On completion of this chapter, the reader will be able to: 1. Describe anatomic changes in the lungs resulting from the normal aging process. 2. Describe age-related changes in ventilation. 3. List nursing diagnoses for older adults with respiratory diseases. 4. Identify nursing interventions and outcomes for older adults with various respiratory alterations. 5. Discuss smoking cessation methods and interventions. 6. Identify risk factors for the development of tuberculosis in older adults. 7. List the benefits of pulmonary rehabilitation for older adults with chronic obstructive pulmonary disease. Normal aging results in changes to the ribs and vertebrae. The ribs become less mobile and chest wall compliance decreases. Osteoporosis and calcification of the costal cartilage lead to increased rigidity and stiffness of the thoracic cage. If kyphosis or scoliosis is present, degeneration of the intervertebral disks occurs, resulting in a shorter thorax with an increased anteroposterior diameter. Advanced cases may result in marked limitation of thoracic movement because of the rib cage resting on the pelvic bones. There is also progressive loss of elastic recoil of the lung parenchyma and conducting airways and reduced elastic recoil of the lung and the opposing forces of the chest wall. The lung becomes less elastic as collagenic substances surrounding the alveoli and alveolar ducts stiffen and form cross-linkages that interfere with the elastic properties of the lungs. Any and all of these structural changes makes it more difficult for the older person to ventilate. It requires more energy. Table 24–1 summarizes various changes in the aging respiratory system. TABLE 24–1 AGE-RELATED CHANGES IN THE RESPIRATORY SYSTEM Modified from Pierson DJ, Kacmarek RM, editors: Foundations of respiratory care, New York, 1992, Churchill Livingstone. As previously described, many of the changes in pulmonary functions in older adults are related to the changes in elastic recoil and musculoskeletal changes of the chest wall. Table 24–2 lists the lung volumes measured, the normal findings, and alterations related to aging. The ability to determine accurate pulmonary function by testing requires patience on the part of the health care provider as an older client may not be able to perform quickly. Ensure adequate time for this assessment of the older adult client. TABLE 24–2 PULMONARY FUNCTION CHANGES IN OLDER ADULTS Increased oxygen demands during exercise periods may well exceed the abilities of older clients, and for those with COPD, activity intolerance is exacerbated. In addition, older clients are more likely to have comorbidities involving the cardiovascular and respiratory systems. Strength and endurance may also be reduced, which leads to increased immobility and increased breathlessness when activity is attempted. Older clients with COPD and immobility may benefit from a program of regular exercise to increase strength and endurance and decrease breathlessness as the respiratory muscles become trained (see Health Promotion/Illness Prevention Box). reviewing preparations for quitting (e.g., removing associated objects like ashtrays), recommending nicotine replacement therapy, providing advice on successful quitting (e.g., avoid constant exposure to other smokers), providing supplemental educational materials, and offering appropriate skills training and support. Finally, the nurse arranges for follow-up (Agency for Health Care Research and Quality [AHCRQ], 2000). with an acute respiratory or cardiac illness, whereas dyspnea on exertion may be related to immobility and respiratory muscle deconditioning. Older clients with COPD may experience dyspnea on exertion initially and dyspnea at rest as the disease progresses (see Evidence-Based Practice: COPD and Dyspnea). Dyspnea is a common complaint in older clients with pulmonary disease. However, older clients usually do not complain of dyspnea until it begins to interfere with their activities of daily living (ADLs) and then only if those activities are important to them. For example, it may become difficult to use the stairs. An older client may simply choose the elevator or escalator and not consider reporting the shortness of breath associated with stair climbing. It is important to determine which ADLs an older client no longer participates in and why. at rest. They may become demanding and controlling in dealing with their families and friends. The quality of older clients’ lives depends on their feelings about and control of the disease. Support groups sponsored by the American Lung Association and local hospitals are available to help clients and families deal with anger, loss of control, and hopelessness. The family or a significant other needs to be included in all aspects of planning and care for an older client with respiratory illness. The client’s success in complying with the medical recommendations may depend on the assistance he or she receives in getting to the physician’s office, getting to the pharmacy for medications, administering medications, and performing ADLs. Older clients with respiratory disease need a good family support system and a health care team to support both them and their families (see Evidence-Based Practice: COPD and Family Dynamics). Asthma is a chronic inflammatory disease that affects the airways and is characterized by reversible airway obstruction, airway inflammation, and increased airway responsiveness to a variety of stimuli. Asthma has higher morbidity and mortality rates in older adults than in other age groups. Older patients diagnosed with asthma have lower expiratory flow rates and fewer symptom-free periods. Because of other comorbidities and the normal deterioration caused by aging, a diagnosis of asthma may be delayed by the provider. Airway inflammation contributes to airway hyperresponsiveness; airflow limitations, including acute bronchoconstriction, airway edema, and mucous plug formation; airway wall remodeling; respiratory symptoms; and disease chronicity (National Heart, Lung, and Blood Institute [NHLBI], 2007a. Inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, often at night or early in the morning. Recent evidence suggests that persistent abnormalities in lung function are associated with subbasement membrane fibrosis in some clients. Patients with asthma, especially older ones who may not have had this disease through most of their lives, require careful education to include self-management, how to adjust medications during exacerbations, and the correct way to prepare themselves for exposure to known triggers. The goals of asthma therapy are to control asthma by reduction of impairment and risk, which can be done by (1) preventing chronic and troublesome symptoms like coughing or breathlessness during the day, at night, or after exercise, (2) maintaining (near) normal pulmonary function, (3) maintaining normal activity levels including exercise and attendance at work or school, (4) requiring infrequent use (≤2 days a week) of short-acting inhaled beta2-agonists and satisfying the client’s and family’s expectations of asthma care, (5) preventing recurrent exacerbations and minimizing emergency department visits, and (6) providing optimal pharmacologic treatment with minimal or no adverse effects (NHLBI, 2007a). The NHLBI (2007a) recommends a stepwise approach to pharmacologic management. The specific drug, dose, and frequency are dictated by the severity of the asthma attack at the time that therapy is initiated, then the drug should be stepped down to maintain long-term control with the minimum medication necessary. Medications are classified into two categories: long-term-control medications and quick-relief medications. Long-term control medications are taken on a daily basis and include antiinflammatory agents, long-acting bronchodilators, and leukotriene modifiers. Corticosteroids are the most potent and effective long-term-control medications in the treatment of mild, moderate, or severe persistent asthma. They are well tolerated and safe when used at the recommended dosage. Most of the benefit is achieved with relatively low doses, and the potential for side effects increases with the dose. However, for asthma not controlled with maintenance doses of corticosteroids, there are now two options. The first is to combine the corticosteroids with long-acting beta2-agonists, and the second, most recent recommendation is to increase the dose of corticosteroids (NHLBI 2007b). The clinical response to corticosteroids is a reduction in airway inflammation, improvement in peak expiratory flow rate (PEFR), diminished airway hyperresponsiveness, prevention of exacerbations, and possible prevention of airway wall remodeling. Corticosteroids are generally inhaled twice a day. Anticholinergics, such as ipratropium bromide (Atrovent), may provide an additive benefit to inhaled beta2-agonists in the treatment of severe exacerbations. They may also be used as an alternative to short-acting beta2-agonists for patients who do not tolerate them well. Finally, systemic corticosteroids, although not short-acting, may be used in the treatment of moderate to severe asthma exacerbations as an adjunct to the short-acting beta2-agonists. Their onset of action is more than 4 hours, and they act by preventing progression of the exacerbation, speeding recovery, and preventing early relapse (NHLBI, 2007b). Step 1: No daily medication indicated. Short-acting beta2-agonists are used as required (prn). If they are used more than two times a week, consider long-term control therapy. Step 2: Daily low-dose inhaled corticosteroid. Step 3: Daily low-dose inhaled corticosteroid used in conjunction with a long-acting bronchodilator. An alternative is to increase the corticosteroid dose to a medium level without the addition of a long-acting bronchodilator. If ineffective, a leukotriene modifier may be added to a low-dose corticosteroid. Short-acting beta2-agonists are used prn. If they are used daily or if there is an increase in use, add additional long-term control therapy. Step 4: Daily antiinflammatory: inhaled corticosteroid (medium dose) and a long-acting bronchodilator. If ineffective, a leukotriene modifier may substitute for the long-acting bronchodilator. Short-acting beta2-agonists are used prn. If they are used daily or if there is an increase in use, add additional long-term control therapy. Step 5: Daily inhaled corticosteroid (high dose) plus a long-acting bronchodilator. Consider an immunomodulator for patients with allergies. Short-acting beta2-agonists are used prn. If they are used daily or if there is an increase in use, add additional long-term control therapy. Step 6: Daily inhaled corticosteroid plus long-acting bronchodilator plus an oral corticosteroid. Consider an immunomodulator for patients with allergies. Asthma management in older adults may coexist with management of chronic bronchitis or emphysema. A trial of systemic corticosteroids is useful in determining the presence of reversible airflow obstruction (NHLBI, 2007a). An older adult may have medical conditions such as cardiac disease and osteoporosis that are aggravated by asthma medications. Older adults with ischemic heart disease may be more sensitive to beta2-agonist side effects such as tremors and tachycardia; the dosage may need to be adjusted, or different medications may need to be added as an adjunct. Corticosteroids may cause confusion, agitation, and changes in glucose metabolism in older adults. The use of inhaled corticosteroids in older adults may predispose them to a reduction in bone mineral content, especially if there is preexisting osteoporosis, changes in estrogen levels affecting calcium utilization, and a sedentary lifestyle. NHLBI (2007a) recommends calcium and vitamin D supplements, as well as estrogen replacement therapy when appropriate. There is also an increased risk for adverse drug and disease interactions: asthma may be exacerbated by the use of nonsteroidal antiinflammatory agents for arthritis, aspirin for circulation, nonselective beta-blockers for hypertension, or glaucoma eye drops that contain beta-blockers. Finally, it is imperative that older adults are carefully assessed for their ability to use prescribed medications appropriately and devices correctly as there is an increased risk of physical (arthritis, visual) or cognitive impairments that could be challenging for them (NHLBI, 2007b). Evaluation of respiratory symptoms includes effect on ADLs, quantity of breathlessness on a scale of 1 to 10 (Stupka & deShazo, 2009), presence of asthma triggers, and frequency of the need for bronchodilator therapy. Physical assessment includes inspection of the chest for shape and symmetry and determination of respiratory rate and pattern, body position, use of accessory muscles of respiration, and amount and color of sputum production. Palpation and percussion of the chest are indicated so that increased tactile fremitus, chest wall movement, and diaphragmatic excursion can be assessed. When the chest wall is auscultated, the older adult should be given enough time to take deep breaths comfortably without becoming dizzy. Determine the presence of any wheezing, the phase of respiration in which it occurs, and whether it is present during a forced expiratory maneuver. Determination of the peak expiratory flow rate (PEFR) with a peak expiratory flow meter (PEFM) is important in determining trends of airway resistance (Fig. 24–1). Nursing diagnoses common for an older client with asthma include (Kaufman, 2007) • Ineffective airway clearance related to bronchospasm, excessive mucus production, tenacious secretions, adventitious breath sounds • Impaired gas exchange related to alveolar–capillary membrane changes • Knowledge deficit: asthma related to lack of information and education about asthma The diagnosis of asthma is based on episodic symptoms of airflow obstruction that are partially reversible. Key indicators for the diagnosis of asthma include (1) wheezing, (2) a history of a cough that is worse at night, (3) recurrent difficulty breathing and chest tightness,(4) variation in PEFR of 20% or more, and (5) symptoms that worsen during exercise, with viral infection, in the presence of environmental irritants such as animal fur, dust mites, mold, smoke, pollen, changes in weather, airborne chemicals, or dust, during menses, or with strong emotional expression (NHLBI, 2007a). Pulmonary function tests (PFTs) are used to measure the presence and amount of airway obstruction. An FEV1/forced vital capacity (FVC) ratio of less than 65% indicates obstruction of airflow. Measurements of FEV1, FVC, and the FEV1/FVC ratio before and after inhaled short-acting bronchodilators are recommended. Other diagnostic procedures include methacholine, histamine or exercise challenge, chest x-ray studies, allergy testing, ear, nose, and throat evaluation for nasal polyps and sinus disease, evaluation for gastroesophageal reflux, a 1- to 2-week evaluation of diurnal variation in PEFR, and evaluation for vocal cord dysfunction (NHLBI, 2007a). The diagnosis and management of asthma in older clients is more difficult than in younger clients. The symptoms of asthma mimic other conditions such as myocardial ischemia or pulmonary embolus (NHLBI, 2007a). Asthma may appear as late as the eighth or ninth decade of life. Older adults with asthma may not show allergic skin sensitivity; therefore serum IgE and eosinophil levels can be more predictive. Incomplete reversibility of airflow obstruction is increasingly common (NHLBI, 2007). Older adult clients with asthma may only achieve a 12% improvement in their FEV1, even with optimally prescribed inhaled bronchodilators (NHLBI, 2007a). In older clients with heartburn, coughing, nocturnal symptoms occurring early in the night, and resistance to routine therapy, gastroesophageal reflux disease should be considered (NHLBI, 2007a). Asthma is classified into three categories according to the severity of symptoms, frequency of nighttime symptoms, and lung function (Table 24–3). Asthma also occurs as seasonal asthma, cough variant asthma, and exercise-induced asthma. TABLE 24–3 CLASSIFICATION OF ASTHMA BY SEVERITY OF DISEASE BEFORE TREATMENT∗ ∗After treatment, severity is measured by the minimum medications needed to maintain good health. From National Heart, Lung, and Blood Institute, National Institutes of Health (NIH). (2007a). Clinical practice guidelines; guidelines for the diagnosis and management of asthma. Retrieved May 2009, from http://www.nhlbi.nih.gov/guidelines/asthma/index.htm. Older clients with asthma and their families should be included in care planning (NHLBI, 2007a). It is important to incorporate the changes in ADLs that are required for ongoing monitoring and maintenance of clients with asthma. Expected outcomes include (Moorhead, Johnson, Maas, & Swanson, 2008) 1. The client will maintain a patent airway. 2. The client will maintain arterial blood gas (ABG) values at baseline. 3. The client will be able to demonstrate proper use of the PEFM. 4. The client will be able to demonstrate relaxation techniques to control breathing. 5. The client will be able to list the significant and reportable signs and symptoms. Interventions for clients with asthma include health maintenance, lifestyle changes, administration of medications at designated time intervals, exercise, and promotion of hydration and good nutrition (McCloskey & Bulechek, 1996). Education is started at the time of diagnosis and is integrated into every aspect of care. Emphasis is placed on asthma self-management; basic facts about asthma; roles of medications; environmental control measures; the use of inhalers, spacers, and PEFMs; and a daily written action plan for management of exacerbations (NHLBI, 2007b). Additional topics include smoking cessation, weight gain or loss, exercise requirements, and breathing retraining. In addition to the basic interventions already described, older clients may require special considerations. The nurse should be accommodating to any neurologic changes such as altered senses, decreased fine motor movements, and memory loss. These expected changes can be managed in various ways. Make treatment plans simple. Use short explanations and easily explained graphs. Make sure instructional materials are in large type, and use color-coded peak flow meter diaries. Increase lighting, and speak in a low pitched clear voice. Have the patient read and then repeat the instructions, and allow sufficient time for instruction, demonstration, and return demonstrations (NHLBI, 2007b). COPD is characterized by progressive airflow limitation that is not fully reversible and, during the course of the disease, lung tissue that becomes abnormally inflamed. The changes manifested include peripheral airway inflammation, airway fibrosis, hypertrophy of smooth muscles, hyperplasia of goblet cells and resultant mucus hypersecretion, and eventually, the destruction of the lung parenchyma (Barnett, 2009). The two reversible components in COPD are airway diameter and expiratory flow rate. COPD is a broad term that describes two obstructive airway diseases: chronic bronchitis and emphysema. Asthma may also be included in COPD, especially if there is a component of airway hyperreactivity; however, it may be difficult to differentiate between the two, especially if a history of cigarette smoking is present (Kaufman, 2007). COPD is a progressive and ultimately fatal disease. The fatality rate for COPD is more than two times as high in men as in women between the ages of 65 and 74 and three times as high between the ages of 75 and 84. There has been an increase in the number of women with COPD since 1991 (Rabe, 2007). This is most likely a result of the increased number of women who smoke. Risk factors for COPD include age, male gender, reduced lung function, air pollution, exposure to secondhand smoke, familial allergies, poor nutrition, and alcohol intake. COPD is often a comorbid factor in deaths from pneumonia and influenza, it accounts for increased physician visits (Mannino, 2002), and it is preventable and treatable. The characteristic symptoms of COPD are chronic and progressive dyspnea, coughing, and sputum production. Chronic coughing and sputum production may precede limits on airflow by many years, which provides a real opportunity for intervention before it becomes a major health problem. It is also possible that airflow limitations may develop without either a chronic cough or excess sputum production (Rabe, 2007). A diagnosis of COPD should be considered based on a history of exposure to tobacco smoke or other occupational irritants and progressive dyspnea, a chronic cough, and chronic sputum production; the diagnosis should then be confirmed with spirometry testing. COPD is staged based on the percent of the predicted value of FEV1 (Table 24–4). TABLE 24–4 STAGING CHRONIC OBSTRUCTIVE PULMONARY DISEASE BY LEVEL OF AIRFLOW Modified from Rabe KF, Hurd S, Anzueto A, et al: Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary, Am J Respir Crit Care Med 176:532–555, 2007. Most patients seek medical treatment because of progressive dyspnea leading to breathlessness and anxiety. Chronic coughing is often the first sign of COPD, but lack of a cough does not rule it out. Initially, chronic coughing is intermittent, and patients may describe “good days and bad days.” As the disease progresses, the cough is present every day. Wheezing and “chest tightness” may vary from day to day and may vary throughout a single day. Once again, an absence of tightness or wheezing does not rule out COPD. Weight loss, anorexia, depression, and anxiety often accompany the pulmonary signs of COPD (Rabe, 2007). The single most important and cost-effective intervention is smoking cessation. Smoking cessation improves FEV1 and helps relieve symptoms. Benefits to smoking cessation include reduction in the number of respiratory infections, improvement in the function of the mucociliary clearance of the lungs, decreased coughing and dyspnea, increased appetite, and decreased sputum production. Older clients with COPD should also avoid secondhand smoke as it can also cause bronchospasm and coughing. There are now many pharmacotherapies available to help the older client quit smoking. Nicotine replacement drugs and some antidepressants (bupropion and nortriptyline) can increase smoking abstinence rates but should be used as part of an overall program of abstinence (Rabe, 2007). These sympathomimetic drugs work by stimulating the beta2-receptors in the lungs, which results in bronchial dilation, increased mucociliary clearance, and possibly increased diaphragmatic function. The drugs may be administered by metered-dose inhaler (MDI) with a spacer or by aerosolized therapy (Fig. 24–2). Beta2-agonists should be used with caution in the older client with ischemic heart disease. Examples of beta2-agonists include albuterol (Proventil, Ventolin), metaproterenol sulfate (Alupent, Metaprel), and pirbuterol acetate (Maxair). Inhaled anticholinergics—ipratropium bromide or oxitropium bromide—are used to treat chronic bronchitis. They work by inhibiting vagal stimulation of the lungs, preventing contraction of the smooth muscle, and decreasing mucous production. A combination of a short acting beta2-agonist and an anticholinergic results in a greater and more sustained improvement than with either drug alone (Rabe, 2007). Inhaled glucocorticosteroids do not reduce the decline of the older adult with COPD, but for those patients with advanced disease (stage 3 or 4), they have been shown to reduce the frequency of exacerbations and improve overall health status. Oral steroids are no longer recommended because they may lead to steroid myopathy, which is associated with muscle weakness and respiratory failure (Rabe, 2007). Steroid therapy may not be well tolerated in older clients. Long-term oxygen therapy increases survival rates and improves hemodynamics, exercise and lung capacity, and mental status. Supplemental oxygen therapy is indicated for clients with a resting Pao2 ≤ 55 mm Hg or a Sao2 ≤ 88% with or without hypercapnia (Fig. 24–3). Oxygen therapy may also be indicated if the client’s Pao2 is between 55 and 60 mm Hg, the Sao2 is 88% or less, or if there is evidence of pulmonary hypertension, peripheral edema, or polycythemia (hematocrit level > 55%). The primary goal of oxygen therapy is to increase the baseline Pao2 to at least 80 mm Hg and the Sao2 to at least 90%.
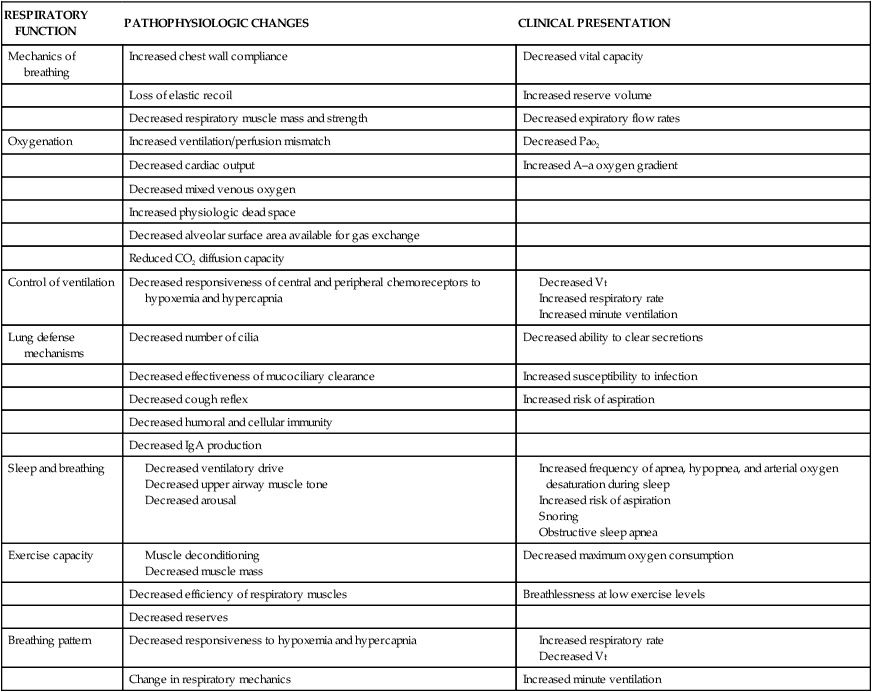
Respiratory Function
Age-Related Changes in Structure and Function
RESPIRATORY FUNCTION
PATHOPHYSIOLOGIC CHANGES
CLINICAL PRESENTATION
Mechanics of breathing
Increased chest wall compliance
Decreased vital capacity
Loss of elastic recoil
Increased reserve volume
Decreased respiratory muscle mass and strength
Decreased expiratory flow rates
Oxygenation
Increased ventilation/perfusion mismatch
Decreased Pao2
Decreased cardiac output
Increased A–a oxygen gradient
Decreased mixed venous oxygen
Increased physiologic dead space
Decreased alveolar surface area available for gas exchange
Reduced CO2 diffusion capacity
Control of ventilation
Decreased responsiveness of central and peripheral chemoreceptors to hypoxemia and hypercapnia
Lung defense mechanisms
Decreased number of cilia
Decreased ability to clear secretions
Decreased effectiveness of mucociliary clearance
Increased susceptibility to infection
Decreased cough reflex
Increased risk of aspiration
Decreased humoral and cellular immunity
Decreased IgA production
Sleep and breathing
Exercise capacity
Decreased maximum oxygen consumption
Decreased efficiency of respiratory muscles
Breathlessness at low exercise levels
Decreased reserves
Breathing pattern
Decreased responsiveness to hypoxemia and hypercapnia
Change in respiratory mechanics
Increased minute ventilation
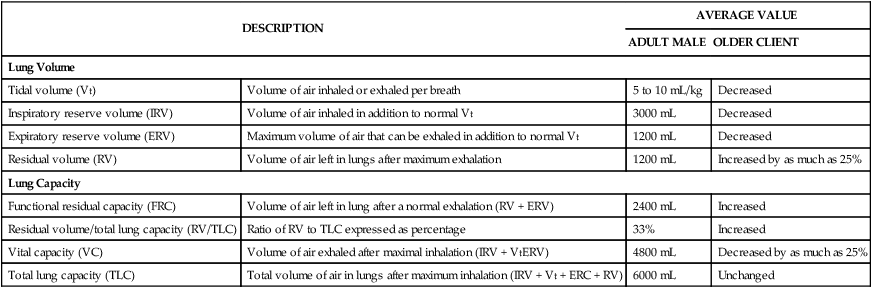
DESCRIPTION
AVERAGE VALUE
ADULT MALE
OLDER CLIENT
Lung Volume
Tidal volume (Vt)
Volume of air inhaled or exhaled per breath
5 to 10 mL/kg
Decreased
Inspiratory reserve volume (IRV)
Volume of air inhaled in addition to normal Vt
3000 mL
Decreased
Expiratory reserve volume (ERV)
Maximum volume of air that can be exhaled in addition to normal Vt
1200 mL
Decreased
Residual volume (RV)
Volume of air left in lungs after maximum exhalation
1200 mL
Increased by as much as 25%
Lung Capacity
Functional residual capacity (FRC)
Volume of air left in lung after a normal exhalation (RV + ERV)
2400 mL
Increased
Residual volume/total lung capacity (RV/TLC)
Ratio of RV to TLC expressed as percentage
33%
Increased
Vital capacity (VC)
Volume of air exhaled after maximal inhalation (IRV + VtERV)
4800 mL
Decreased by as much as 25%
Total lung capacity (TLC)
Total volume of air in lungs after maximum inhalation (IRV + Vt + ERC + RV)
6000 mL
Unchanged
Factors Affecting Lung Function
Exercise and Immobility
Smoking
Smoking Cessation
Respiratory Symptoms Common in Older Clients
Respiratory Alterations in Older Clients
Obstructive Pulmonary Disease
Asthma
Treatment
Long-term control medications
Quick-relief medications
Asthma medications administered through a stepwise approach
Nursing Management
![]() Assessment
Assessment
![]() Diagnosis
Diagnosis
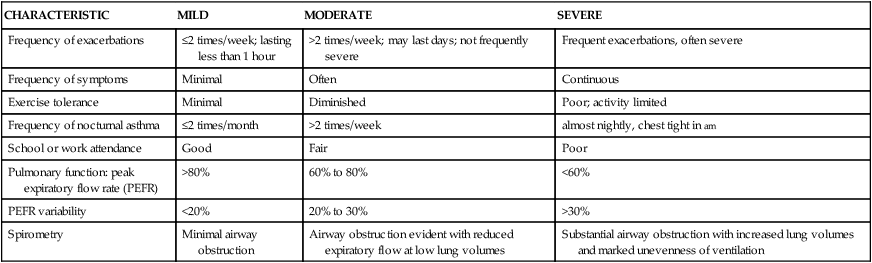
CHARACTERISTIC
MILD
MODERATE
SEVERE
Frequency of exacerbations
≤2 times/week; lasting less than 1 hour
>2 times/week; may last days; not frequently severe
Frequent exacerbations, often severe
Frequency of symptoms
Minimal
Often
Continuous
Exercise tolerance
Minimal
Diminished
Poor; activity limited
Frequency of nocturnal asthma
≤2 times/month
>2 times/week
almost nightly, chest tight in am
School or work attendance
Good
Fair
Poor
Pulmonary function: peak expiratory flow rate (PEFR)
>80%
60% to 80%
<60%
PEFR variability
<20%
20% to 30%
>30%
Spirometry
Minimal airway obstruction
Airway obstruction evident with reduced expiratory flow at low lung volumes
Substantial airway obstruction with increased lung volumes and marked unevenness of ventilation
![]() Planning and Expected Outcomes
Planning and Expected Outcomes
![]() Intervention
Intervention
Chronic Obstructive Pulmonary Disease
Signs and Symptoms
Diagnostic Tests and Procedures
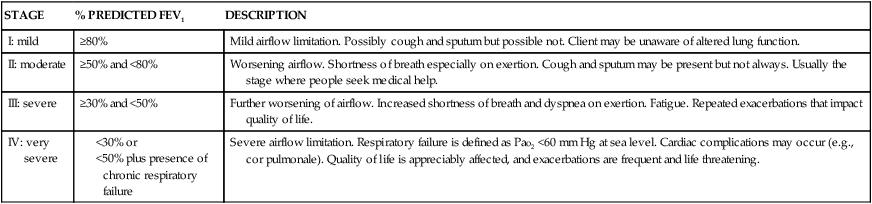
STAGE
% PREDICTED FEV1
DESCRIPTION
I: mild
≥80%
Mild airflow limitation. Possibly cough and sputum but possible not. Client may be unaware of altered lung function.
II: moderate
≥50% and <80%
Worsening airflow. Shortness of breath especially on exertion. Cough and sputum may be present but not always. Usually the stage where people seek medical help.
III: severe
≥30% and <50%
Further worsening of airflow. Increased shortness of breath and dyspnea on exertion. Fatigue. Repeated exacerbations that impact quality of life.
IV: very severe
Severe airflow limitation. Respiratory failure is defined as Pao2 <60 mm Hg at sea level. Cardiac complications may occur (e.g., cor pulmonale). Quality of life is appreciably affected, and exacerbations are frequent and life threatening.
Treatment
Bronchodilators
Beta2-agonists
Anticholinergics
Glucocorticosteroids
Oxygen therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





