CHAPTER 14
Renovascular Disease and Ischemic Nephropathy
L. Beth Sossoman
First Edition Authors: Maria Henderson-Everhardus and J. Hal. Van Bockel
OBJECTIVES
1. Identify the components of a comprehensive physical examination (including clinical clues for proceeding to diagnostic testing) for people with renovascular hypertension.
2. Perform a comprehensive renovascular health assessment.
3. List the appropriate diagnostic tests relevant to renovascular hypertension.
4. Discuss the appropriate medical, radiological, and surgical interventions relevant to renovascular hypertension.
5. Identify the nursing implications of renovascular hypertension.
Introduction
Renovascular disease (RVD) encompasses atherosclerotic, fibromuscular, and inflammatory diseases. RVD can lead to renovascular hypertension (RVH) which causes reduced kidney perfusion and renal ischemia (Fenves & Ram, 2006). Hypertension affects an estimated 78 million American adults (Go et al., 2013). It is estimated that RVH accounts for 5% of all cases of hypertension. This accounts for upward of 4 million people (Hartman & Kawashima, 2009). Renal artery stenosis (RAS) may be a cause of RVH. RAS has been found to be associated with atherosclerosis in other vascular beds (i.e., carotids and peripheral vessels) and is associated with adverse coronary events (Hirsch et al., 2006). RAS is caused by narrowing of the artery resulting predominately from either atherosclerosis or fibromuscular dysplasia (FMD). Diminished perfusion to the kidney results in increased renin secretion and production of angiotensin II. This results in systemic vasoconstriction and retention of salt and water. If RAS leads to renal impairment, the long-term outcome is poor (Hirsch et al., 2006).
The etiology of renal vascular disease may affect one or more vessels in one or both of the kidneys. The most common cause of RVH is atherosclerosis, which is seen in 90% of cases (Plouin & Bax, 2010). This is primarily seen in people over 50 years of age, especially men, smokers, with a history of treatment-resistant hypertension, and atherosclerosis in other blood vessels (Colyer, Eltahawy, & Cooper, 2011). RVH caused by fibrodysplastic RAS occurs primarily in children, young adults, and in women of childbearing age (Colyer et al., 2011).
The natural history of RVH differs by the cause of RAS. Arteriosclerotic RAS (ARAS) is characterized by intimal thickening, subintimal lipid deposition, progressive narrowing, and finally occlusion of the arterial lumen. Atherosclerotic lesions usually occur at the ostium of the renal artery or in the proximal one third of the vessel. ARAS is progressive and is manifested with high-grade lesions (between 50% and 70% depending on the measurement methodology) and systemic hypertension (usually defined as a systolic pressure >160 mm Hg). Renal insufficiency/failure is associated with atherosclerotic RAS when coupled with an abnormal baseline creatinine and bilateral stenosis (Dworkin & Cooper, 2009). ARAS is also associated with increased mortality and morbidity, usually from cardiovascular disease (Edwards, Craven, Burke, Dean, & Hansen, 2005; Hirsch et al., 2006). Manifestations on echocardiogram are left ventricular hypertrophy and diastolic dysfunction (Chrysochou & Kalra, 2010).
FMD is classified into three categories: intimal fibroplasia, medial fibroplasia, and perimedial dysplasia. Medial fibroplasia is the predominant type making up 85% of cases and is characterized by the typical “string of pearls” on angiographic studies (Hirsch et al., 2006). Other arteries can be affected including the carotid, vertebral, iliac, and mesenteric arteries (Coffman, Falk, Molitoris, Nelson, & Schrier, 2013). FMD may also be familial in 10% of cases. Complications, which result from the dysplastic process, include renal artery aneurysms, stenosis, dissections, and thrombosis (Colyer et al., 2011).
Either type of RAS can lead to a reduction of renal parenchymal blood flow sufficient to provoke sustained renin production, which may result in hypertension (RVH). If only one kidney is affected, the renin–angiotensin–aldosterone system is activated, causing renin-dependent hypertension (Hirsch et al., 2006). If both kidneys are affected or only a solitary kidney is present, renin production is inhibited and hypertension becomes more fluid dependent, which results in cardiac failure from fluid overload (Chrysochou & Kalra, 2010). Having bilateral RAS is associated with a higher mortality rate (Rocha-Singh et al., 2008). In both types of RAS, renal impairment and ischemic nephropathy, defined as a decrease in glomerular filtration rate (GFR) may result (Elliott, 2008). Intervention with either surgery or catheter-based technology may or may not improve one’s hypertension, serum creatinine, or GFR.
 I. Anatomy (see Fig. 14-1)
I. Anatomy (see Fig. 14-1)
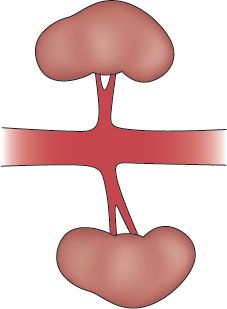
FIGURE 14.1 Normal renal vasculature and kidneys.
A. The Kidneys are located in the retroperitoneal space (posterior to the abdominal organs) on either side of the aorta and vena cava.
B. The Adult Kidney is 115 to 117 g in weight, 9 to 11 cm long, 5 to 7 cm wide, and 2 to 3.5 cm thick.
C. The Right Kidney is 1 to 2 cm lower than the left kidney because of the liver (Jarvis, 2012).
D. Kidney Size Varies by Sex and Age.
1. Adult women have slightly smaller kidneys than adult men.
2. Infants and young children have proportionately smaller kidneys than adults.
E. The Blood Supply of the kidneys arises from the abdominal aorta.
F. The Renal Artery enters the kidney at the renal hilus.
G. Duplicate or Multiple Arteries are frequently found.
H. The Main Renal Artery bifurcates into several segmental branches, which progressively branch within the kidney into the lobar, arcuate, and interlobular arteries.
I. Interlobular Arteries are located in the outer zone of the kidney (cortical layer) and give rise to the afferent arterioles, which supply the glomerulus and the nephrons.
J. The Renal Vein is paired with the renal artery and is connected to the inferior vena cava.
 II. Pathophysiology (see Fig. 14-2)
II. Pathophysiology (see Fig. 14-2)
A. RAS (from atherosclerotic occlusive disease)
1. ARAS usually occurs at the ostium and proximal one third of the main renal artery, as well as the nearby aorta (Colyer et al., 2011).
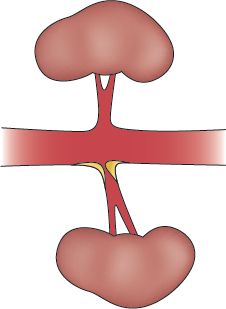
FIGURE 14.2 Unilateral renal artery ostial stenosis.
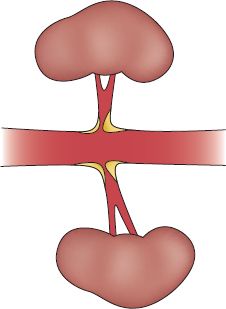
FIGURE 14.3 Bilateral renal artery ostial stenosis.
2. Changes from arteriosclerosis lead to intimal thickening and subintimal lipid deposition, which gradually narrows and eventually occludes the arterial lumen.
3. Stenosis or occlusion can be unilateral or bilateral (see Figs. 14-3 and 14-4).
4. As the renal artery narrows, the kidney becomes ischemic, atrophied, fibrotic, and ultimately, shrinks.
5. Decreased pressure in the arterioles stimulates the juxtaglomerular apparatus to increase renin secretion, leading to RVH (Hallett, Mills, Earnshaw, & Reekers, 2004).
6. With stenosis or occlusion of the renal artery, the kidney ultimately suffers ischemia or infarction.
B. FMD
1. FMD is commonly found in the middle and distal two thirds of renal arteries (Hirsch et al., 2006).
2. FMD causes defects in the medial portion of the artery (Plouin & Bax, 2010).
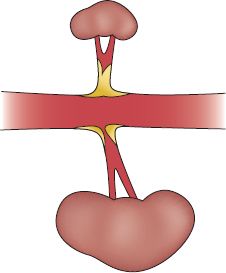
FIGURE 14.4 Unilateral renal artery ostial stenosis and contralateral occlusion with a shrunken kidney.
3. It rarely causes intimal hyperplasia or adventitial fibroplasia (Plouin & Bax, 2010).
4. FMD commonly impacts small and mid-sized arteries. Most commonly affected arteries are renal (60% to 75% of cases) and cerebral (25% to 30% of cases) (Coffman et al., 2013).
5. FMD rarely results in renal artery occlusion or ischemic nephropathy.
6. FMD can lead to development of saccular aneurysms, microaneurysms, and stenosis.
C. Renovascular Hypertension (see Fig. 14-5) (Hallett et al., 2004; Munden, 2007)
1. Blood pressure is controlled by the renin–angiotensin–aldosterone mechanism.
2. RVH occurs when the renin–angiotensin system is activated secondary to RAS.
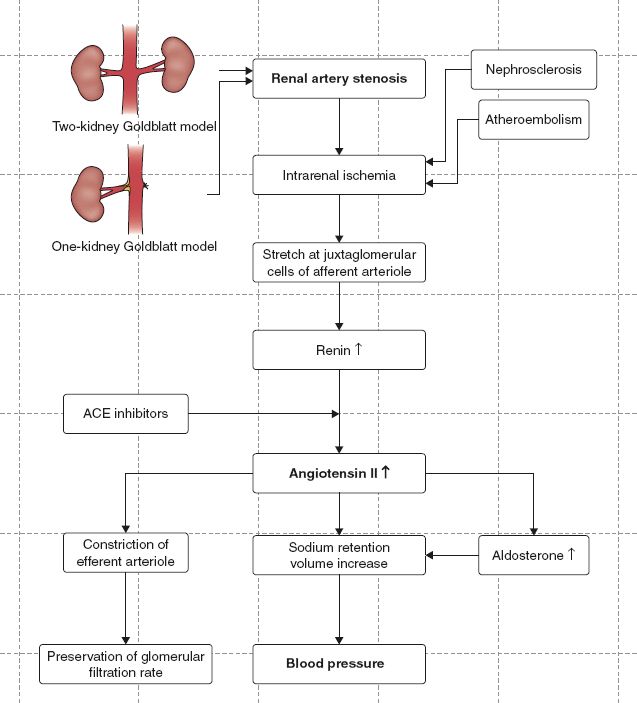
FIGURE 14.5 Pathophysiologic algorithm of renovascular hypertension.
3. When blood pressure within the kidneys falls, the kidneys release renin into the bloodstream.
4. Renin and angiotensinogen, in the liver, combine to form angiotensin I.
5. Angiotensin I is converted to angiotensin II in the lungs.
6. Angiotensin II causes vasoconstriction, increased peripheral resistance, and elevated blood pressure.
7. Angiotensin II has direct renal actions causing reabsorption of sodium and water.
8. Angiotensin II stimulates the adrenal gland to release aldosterone.
9. Aldosterone causes the kidneys to retain sodium and water.
10. Sodium in the kidney causes retention of water.
11. Extra fluid retention expands the volume of blood in the body, leading to increased blood pressure.
 III. Etiology
III. Etiology
A. Arteriosclerosis
1. Arteriosclerosis is the cause of RVH in approximately 90% of cases (Hirsch et al., 2006).
2. In arteriosclerosis, intimal thickening and subintimal lipid deposition leads to artery occlusion. The disease process is progressive.
3. Arteriosclerosis and the resulting stenosis or occlusion increases the risk of renal failure.
B. FMD
1. It is the second most common cause of RVH. It is seen in 10% of cases (Coyler et al., 2011).
2. FMD is not an inflammatory or atherosclerotic process (Hirsch et al., 2006)
C. Other Causes (Coyler et al., 2011; Plouin & Bax, 2010)
1. Takayasu arteritis: form of systemic vasculitis. Found most commonly in China, Japan, and India
2. Renal artery aneurysms: seen in saccular forms at the bifurcation of the renal artery
3. Embolism: either spontaneous or occurring during vascular procedures
4. Renal artery occlusion during endovascular procedures for aortic aneurysm repair (EVAR) (Coffman et al., 2013)
5. Aortic dissection
6. Polyarteritis nodosa: a form of vasculitis causing aneurysms
7. Arteriovenous fistula
8. RAS after transplantation
 IV. Complications
IV. Complications
A. Of Atherosclerosis
1. Hypertension, including refractory hypertension (Plouin & Bax, 2010)
2. Renal dysfunction (Plouin & Bax, 2010). Most common cause of end-stage renal disease in individuals over the age of 60 on renal replacement therapy (Hallett et al., 2004)
3. Renal atrophy or injury (Hirsch et al., 2006)
4. Pulmonary edema (Plouin & Bax, 2010)
B. Of FMD (Plouin & Bax, 2010)
1. Renal artery dissection
2. Acute renal infarction
3. Rarely causes renal function impairment
 V. Assessment
V. Assessment
A. Risk Factors
1. Race: more prevalent in Caucasians than African Americans
2. Age
a. 15 to 30 years of age (FMD)
b. 55 years of age (arteriosclerotic disease)
3. Arteriosclerotic disease of the heart and/or peripheral blood vessels (Chrysochou & Kalra, 2010)
4. Smoking (current or previous history)
5. Hypertension (Hirsch et al., 2006)
a. Onset before age 30
b. Onset of severe hypertension after age 55
c. Accelerated hypertension (sudden/persistent worsening of previously controlled hypertension)
d. Resistant hypertension (on full dose of three antihypertensive agents including a diuretic and not achieving target blood pressure)
e. Malignant hypertension (hypertension with end-organ system damage)
B. Patient History (McLaughlin, Jardine, & Moss, 2000)
1. RVH resulting from FMD
a. Subjective findings
1) Asymptomatic
2) Usually discovered by physical examination with elevation of blood pressure
b. Objective findings
1) History of etiologic factors
2) Bilateral retinopathy (grade III or IV) on fundoscopic examination
3) Aortic-renal bruit usually heard midline or toward flank
4) Elevated bilateral arm blood pressure with diastolic hypertension >110 mm Hg
5) Higher nocturnal blood pressures (Coffman et al., 2013)
2. RVH resulting from arteriosclerotic disease
a. Subjective findings
1) Fatigue
2) Visual changes
3) Headache
4) Pain from coronary artery disease, peripheral artery disease, and ischemia
5) Peripheral edema
6) Inability to regulate blood pressure with medications
7) Pulmonary edema and wheezing (rare, but can occur)
b. Objective findings
1) History of etiologic factors
2) Bilateral retinopathy (grade III or IV) on fundoscopic examination
3) Aortic-renal bruit
4) Flank pain present with severe kidney ischemia
5) Decreased peripheral pulses with CAD and PAD
6) Bilateral arm blood pressure readings elevated, systolic >160 mm Hg
7) Higher nocturnal blood pressures (Coffman et al., 2013)
C. Physical Examination
1. Inspection
a. Venous distention or peripheral swelling
b. On fundoscopic examination, arteriolar narrowing, arteriovenous nicking, hemorrhage, exudates, or papilledema (grade III or IV)
c. Peripheral edema
2. Palpation
a. Decreased or absent aortic and peripheral pulses (indicative of peripheral artery disease)
b. Jugular venous distention and central venous pressure elevation
c. Peripheral edema
d. Percussion
e. Flank tenderness over kidneys
f. Pulmonary edema
3. Auscultation
a. Bruits in aorta, carotid, renal, and femoral arteries
b. Changes in bilateral arm blood pressures with change in position (sitting, lying, and standing)
c. Elevated blood pressure
d. Abnormal breath sounds resulting from pulmonary edema
 VI. Considerations Across the Life Span
VI. Considerations Across the Life Span
A. Children and Young Adults
1. Suspect RVH in children and young adults presenting with sudden onset severe hypertension.
2. Take blood pressure at every visit to monitor for any sudden elevations in blood pressure.
3. Evaluate flank pain, if present
a. Flank pain is most often the result of an infection, but in some cases may be due to renal ischemia.
b. Renal artery disease should be ruled out if RVH is suspected.
4. Clinical clues for a high level of suspicion for RVH
a. Age <25 years at onset of hypertension
b. Sudden onset of hypertension in the absence of family history of hypertension
c. Onset of moderate to severe hypertension (defined as BP >160/100) develops into malignant hypertension
d. Headaches
e. Resistance to standard pharmacological management
B. Women of Childbearing Age
1. Have a higher risk of developing fibrodysplastic RAS than atherosclerotic RAS
2. Suspect RVH in cases of abrupt onset hypertension or treatment-resistant hypertension
3. Clinical clues same as for children and young adults
C. Adult/Elderly
1. People >50 years are more likely to have arteriosclerotic disease
2. Monitor blood pressure and renal function regularly, in anyone with CAD, PAD, or renal disease
3. Clinical clues for a high level of suspicion for RVH (Harvin et al., 2012)
a. Abdominal bruit
b. Malignant or accelerated hypertension
c. Significant (diastolic pressure >110 mm Hg) in a young adult (<35 years old)
d. New onset after the age of 50 (or age 55, depending on the source)
e. Sudden development or worsening of hypertension
f. Refractory hypertension
g. Deterioration of GFR in response to ACE inhibitors or angiotensin receptor blocking agents
h. Generalized arteriosclerosis in other major organs
i. Atrophy of one kidney or discrepancy of more than 1.5 cm between the kidneys (Hirsch et al., 2006)
j. Unexplained pulmonary edema (Hirsch et al., 2006)
 VII. Diagnostic Testing
VII. Diagnostic Testing
A. General Information
1. ACC/AHA (Hirsch et al., 2006) recommends one of the following for detection of RAS: duplex ultrasonography, computerized tomographic angiography (CTA, in patients with normal renal function), and MRA.
2. If there is a high level of clinical suspicion and the noninvasive tests are inconclusive, then invasive catheter angiography is indicated (Hirsch et al., 2006).
3. The index of suspicion for RVH is based on clinical clues, as outlined above.
4. Calculations of sensitivity, specificity, and accuracy are usually based on a gold standard comparison like angiography with standard definitions. However, there is no clear definition of significant RAS. Based on the study, the definition of significant RAS can be based on the degree of stenosis (ranging from 50% to 75%) or improvement in blood pressure post intervention (Harvin et al., 2012).
B. Anatomical Studies for RVD
1. Renal duplex ultrasound (Hallett et al., 2004)
a. Description
1) First-line screening test
2) Direct visualization of the renal arteries (B-mode) and hemodynamic blood flow measurements (Doppler) to evaluate renal artery blood flow, parenchymal blood flow, and kidney size
b. Interpretation of findings
1) Normal kidney length: 9 to 11 cm
2) Size difference between kidneys <1.5 cm
3) Criteria that correlates with >60% stenosis (Harvin et al., 2012; Hirsch et al., 2006)
a) Direct peak velocity >180 to 200 cm/sec (higher velocity correlates with a greater pressure differential across the stenosis)
b) Renal to aortic velocity ratio (RAR) >3.5 is indicative of stenosis >60%
c) Rise time >0.07 seconds
d) Difference in renal or segmental resistive index >0.15
c. Advantages
1) Poses no nephrotoxic risk
2) Noninvasive test that visualizes the kidney, renal arteries, perfusion through the kidney
3) May be able to determine bilateral or unilateral stenosis at the origin of the renal artery
4) Requires fasting before testing
5) Can also use bowel prep, like a colonoscopy to improve visualization by reducing colonic contents (Elliott, 2008)
6) Medications do not affect the outcome of the study
d. Disadvantages
1) Technique is operator dependent
2) Even with overnight fasting, the renal arteries are difficult to image because of abdominal artifacts such as obesity, bowel gas, and anatomical location
3) Does not reliably identify renal artery, branch, or polar renal artery disease
4) Can overlook small accessory arteries
e. Sensitivity/specificity (Harvin et al., 2012)
1) Varies by published report
2) 85% to 90% sensitive; however, some investigators have reported sensitivity of 0% (that correlated with a >60% stenosis) as listed above
3) 95% specific
2. CTA (Hirsch et al., 2006)
a. Description
1) Used to evaluate soft tissue and blood flow by taking multiple computerized x-ray photos of the kidney (with the addition of a contrast agent, blood flow can be evaluated).
2) Spiral images are obtained with rotation of the x-rays to reconstruct three-dimensional images (requires iodinated contrast).
3) Densities of various tissues are visualized to determine if stenosis or aneurysms are present.
b. Interpretation of findings (normal)
1) Blood flow is normal throughout the aorta, renal arteries, and renal branches.
2) Vessel lumen size is normal without obstructions or aneurysms present.
c. Advantages
1) Correlates very well with digital subtraction angiography (DSA)
2) Can visualize the kidney in multiple planes and determine any ostial lesions or multiple accessory vessels
3) Visualization of renal tumors and cysts, ureteral obstructions, calculi, and abnormalities
4) Can use in patients with metal stents and can assess for in-stent restenosis
d. Disadvantages
1) Allergic reactions or acute renal toxicity resulting from contrast agent
2) High cost
3) Contraindicated in patients with claustrophobia
e. Sensitivity/Specificity
1) 59% to 96% sensitive
2) 82% to 99% specific
3. Magnetic resonance imaging/angiography (MRA) (Hirsch et al., 2006)
a. Description
1) Radiofrequency signals are passed through a magnetic field and behavior of hydrogen atoms in the field is recorded
2) Gadolinium, a contrast agent with low nephrotoxicity, is used to detail the structures and blood flow of the aorta and renal arteries
a) Agent has to be used with caution in patients with stage III or IV chronic kidney disease; can cause nephrogenic systemic fibrosis (Coyler et al., 2011)
b) Blood flow and vessel lumens are visualized
b. Interpretation of findings (normal)
1) Blood flow is normal throughout the aorta, renal arteries, and renal branches
2) Vessel lumen size is normal without obstructions or aneurysms present
c. Advantages
1) Good visualization of ostial, proximal, and distal renal lesions that are atherosclerotic
2) No need for nephrotoxic contrast
d. Disadvantages
1) Preparation and time of procedure
2) High cost
3) Contraindicated for patients with metal implants
4) Contraindicated in patients with claustrophobia
5) Limited availability
e. Sensitivity/specificity
1) 90% to 100% sensitive
2) 76% to 94% specific
3) Reduced accuracy in small branch or distal renal arteries
4) Tendency to overestimate moderate stenosis (40% to 69%)
4. Angiography, intra-arterial DSA, intravascular Doppler ultrasound (IVUS) (Hallett et al., 2004)
a. Description
1) Renal arteriography, can be combined with intra-arterial pressure measurements and IVUS
2) Provides accurate identification and location of soft tissue organs and blood vessels and size and contour of lumens
3) Ultrasound is sound waves which detect the speed of blood cells, determining the flow of blood and noting any turbulence or obstruction
b. Interpretation of findings
1) Normal
a) Kidneys are 9 to 11 cm in length
b) Origin of the aorta and renal arteries are patent without calcification, aneurysm, or obstruction
c) Blood flow within the artery is regular without turbulence noted in the lumen
2) Abnormal
a) Unilateral or bilateral stenosis at the origin of the renal artery (atherosclerosis) or in the main renal artery or its extrarenal or intrarenal branches
b) May include (micro)aneurysms or dissections
c. Advantages
1) Balloon dilatation or stenting can occur in the same setting
2) Can be done in the same setting as a coronary/peripheral arteriography
d. Disadvantages
1) Allergic reactions or acute renal toxicity resulting from contrast agent
2) Dehydration makes patients more susceptible to contrast-induced renal failure, especially in patients with impaired renal function and patients with diabetic nephropathy
3) Contrast-induced renal failure
4) Atheroembolization resulting from catheter manipulations
5) Pseudoaneurysm, hemorrhage, and hematoma may result from femoral puncture
C. Functional Studies for RVD (performed when the patient exhibits hypertension and has unilateral RVD)
1. Currently, there is not a test that can conclusively establish the functional significance of a lesion or predict the response to revascularization
2. Renal venous renin (Harvin et al., 2012; Hirsch et al., 2006)
a. Description
1) Helps to identify functionally significant lesions (Zarins & Gewertz, 2005)
2) Not indicated as an initial screening test
b. Interpretation of findings
1) In patients with unilateral RAS, the ischemic kidney secretes increased renin and the contralateral kidney has relative suppression of renin release
2) With bilateral RAS, the renal vein with the higher renin level has the greatest degree of stenosis (there may be no lateralization of renin levels due to both kidneys being hyper-reninemic)
3) A 1.5:1 renal vein/renin ratio for the involved versus uninvolved kidney can be considered positive for renovascular lesion
c. Advantages—Has been used to select patients for arteriography
d. Disadvantages
1) High rate of false-negative and false-positive results
2) Operator sensitive as it relates to specimen collection
3) Patient hydration is key to accurate results
e. Sensitivity/specificity (Harvin et al., 2012)
1) 72% to 92% sensitive
2) 29% to 42% specific
3. Captopril-augmented nuclear renography (Hartman & Kawashima, 2009)
a. Description (Hirsch et al., 2006)
1) ACC/AHA does not recommend this for initial diagnosis
2) This is a functional assessment of renal perfusion and function
3) Baseline plasma renin level is drawn
4) An ACE inhibitor, captopril, is given in a single oral dose
5) Plasma renin assays are repeated at 60 minutes
6) Blood pressure measurements are repeated at 15-minute intervals of a total of 60 minutes
7) Scintigraphic images and time–activity curves are measured after the injection to evaluate the degree of renal update before and after administration of captopril
b. Interpretation of findings
1) When the renin–angiotensin–aldosterone system is blocked, blood pressure falls
2) Normal finding shows a rapid uptake, followed by excretion of the contrast agent. Fifty percent of the contrast should be excreted in 10 minutes
3) Abnormal findings show delayed uptake and excretion, indicating RAS
c. Advantages
1) Only used in persons with unilateral stenosis when RVH is predominantly the result of inhibition of the renin–angiotensin–aldosterone system
2) Best identifies patients who will likely have improvement in blood pressure after revascularization
3) May be helpful in borderline lesions
d. Disadvantages
1) Not reliable if renal insufficiency and intrinsic parenchymal disease present
2) Not valid for patients with elevated creatinine levels (>2.0 mg/dL)
3) If stenosis is bilateral, ACE inhibitors are effective because there is not enough fluid to affect the renin–angiotensin–aldosterone system
e. Sensitivity/specificity
1) 81% sensitive
2) 59% specific (see Fig. 14-6)
D. Laboratory Tests
1. Blood urea nitrogen (BUN)
a. Description—Provides indirect and rough measure of renal function and GFR, if normal liver function exists
b. Interpretation of findings
1) Normal (Dunning & Fischbach, 2011)
a) Children: 5 to 18 mg/dL or 1.8 to 6.4 mmol/L
b) Adults: 7 to 18 mg/dL or 3.6 to 7.1 mmol/L (SI units)
c) Elderly: 8 to 20 mg/dL or 2.9 to 7.5 mmol/L
2) Abnormal—Increased BUN, indicating a drop in GFR
c. Advantages—Used to establish baseline values for evaluating abnormal function
d. Disadvantages
1) Accurate only if liver function normal
2) Changes in protein intake and hydration may affect BUN levels
2. Serum creatinine
a. Description
1) Used to estimate clearance of creatinine, which is excreted entirely by the kidneys
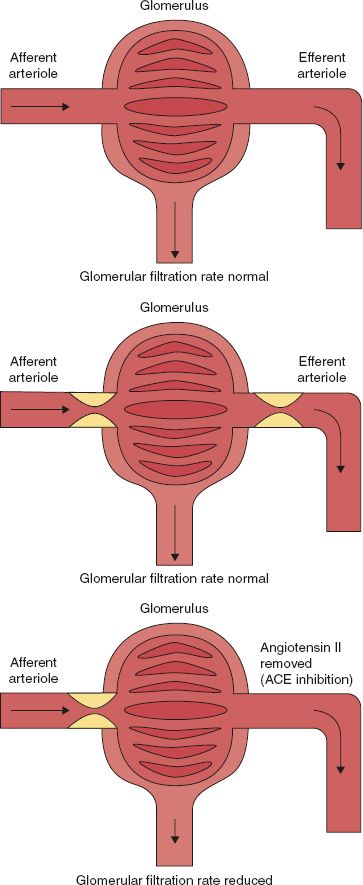
FIGURE 14.6 Glomerular function (top). There is a normal supply of blood without any pressure-reducing stenosis or occlusion in the efferent arteriole resulting in a normal perfusion pressure and a normal glomerular filtration. No compensation of the efferent arteriole is required to maintain the perfusion pressure (middle). Diagram of glomerulus in the presence of a renal artery stenosis (afferent arteriole). This results in a reduced perfusion pressure, which might result in a reduced filtration rate. However, autoregulation resulting in vasoconstriction of the efferent arteriole by angiotensin II results in maintaining the perfusion pressure. This results in a normal glomerular filtration (bottom). The same as in the middle figure. However, high blood pressure is treated by an angiotensin-converting enzyme inhibitor. This results in prevention of the vasoconstrictive effect of angiotensin II on the efferent arteriole. As a result, vasoconstriction of the efferent arteriole is released resulting in a reduction of the perfusion pressure and, consequently, in a reduced glomerular filtration rate. This is usually clinically observed (in particular in the presence of a monokidney) as an increase of the serum creatinine level, reduced perfusion pressure on a renal scan, or a decrease in the volume of the urine production.



