CHAPTER 20
Pulmonary Embolism
Michelle Tinkham
Patrica A. Lewis
First Edition Authors: Patricia A. Lewis and Karla Mees
OBJECTIVES
1. List three risk factors for pulmonary embolism.
2. Discuss methods of prevention of venous thromboembolism.
3. Describe pertinent diagnostic testing modalities for pulmonary embolism.
4. Identify key elements in educating the patient who is treated for pulmonary embolism.
Introduction
A venous embolus is a detached blood clot or thrombus that travels through the venous circulation, generally lodging in a more proximal vessel or site distant from the original source. A pulmonary embolus (PE) is a thrombus that lodges in a segment of the pulmonary arterial system, thereby causing either complete or partial obstruction of pulmonary blood flow that may result in a significant decrease of oxygenation. Most pulmonary emboli originate in the veins of the lower extremities in the form of deep venous thrombosis (DVT), but other sources include the pelvic veins and the right side of the heart. Pulmonary infarction is the term used to describe a local area of necrosis in lung tissue resulting from vascular obstruction. This is an uncommon complication of PE, although some studies have shown that pulmonary infarction is more likely when the lung is already infected or congested prior to the development of pulmonary embolism (Roach & Laufman, 1955).
Pulmonary embolism is the third leading cause of death from cardiovascular disease, exceeded only by ischemic heart disease and stroke, and may be the most common preventable cause of death in the world (Wheeler & Anderson, 1996). Twenty per cent of DVT cases lead to fatal pulmonary embolism (Rosendaal, 1999). The 7-day survival rate for those diagnosed with pulmonary embolism is 59% (Heit et al., 2001). Although true incidence of the disease is unknown because of low autopsy rate and lack of diagnoses, 94,000 cases of pulmonary embolism are identified annually (Heit et al., 2001). ‘‘Most patients with DVT will develop pulmonary embolism and the majority of cases will be clinically unrecognized. Untreated, approximately one third of patients who survive an initial pulmonary embolism will die from a future embolic episode. This is true whether the initial (clot) is small or large. Most patients who die from pulmonary embolism have not had any diagnostic workup, nor have they received any prophylaxis for the disease’’ (Feied & Handler, 2000). It is estimated that the overall financial burden of pulmonary embolism is over $1.5 billion/year in associated health care costs or $30,000 per incident whereas the preventative costs are only $3,000 per patient (American Thoracic Society, 2010).
However, even though the reported number of cases of pulmonary embolism is significantly less than the true incidence, there has been a documented 37% decline in nonfatal PE while at the same time diagnosis of DVT has increased by 108% (Wheeler & Anderson, 1996). This is thought to be due to improved diagnosis and treatment of DVT, with wider acceptance of prophylaxis and public education regarding prevention.
 I. Anatomy
I. Anatomy
A. Pulmonary Artery
1. Composed of elastic connective tissue, smooth muscle, and fibrous connective tissue
2. Large elastic vessel with thinner muscle layer (media) than systemic circulation
3. Originates at right ventricle
4. Lower pressure and resistance than systemic circulation; average pulmonary artery pressure +25/10 mm Hg, and mean of 15 mm Hg
5. Carries unoxygenated blood to the lungs
6. Divides and enters the lung at the hilus with each main bronchus and branches with the bronchus at every division (see Fig. 20-1)
7. Facilitates gas exchange, delivers nutrients to lungs, filters out clots, air, and other debris (McCance & Heuther, 1998)
B. Large Size of inferior vena cava and right heart chambers prevents emboli from adhering to vessel wall prior to pulmonary circulation
 II. Pathophysiology
II. Pathophysiology
A. Direct Consequence of DVT (refer to description in Chapter 19, Superficial Thrombophlebitis and Deep Vein Thrombosis)
1. 90% from deep veins of legs as demonstrated by Virchow through dissection studies (Wheeler & Anderson, 1996)
2. 10% from inferior vena cava, upper extremity, and right side of heart
3. Most common site of origin is iliofemoral system (Abrams, 1997)
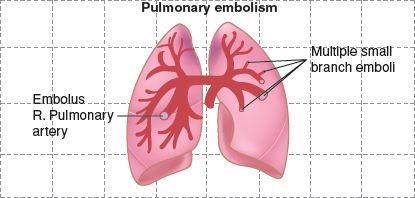
FIGURE 20.1 Pulmonary embolism and infarction.
4. Superficial thrombophlebitis (STP) generally does not lead to PE unless thrombus extends from greater saphenous vein (GSV) beyond saphenofemoral junction (SFJ) into common femoral vein
B. Nonthrombotic Emboli: <10% of PEs
1. Air embolism
a. Displaces blood in the arterioles and capillaries
b. Leads to ischemia and necrosis
2. Amniotic fluid
a. Displaces blood and reduces oxygen, nutrient, and waste exchange
b. Introduces antigens, cells, and proteins
c. Causes inflammation and coagulation
3. Bacterial/septic embolism
a. Subacute bacterial endocarditis the most common cause
1) Vegetation is dislodged from infected valve and injected into circulation
4. Fat embolism
a. Trauma induced, often fracture of large long bone (femur)
1) Globules of fat/bone marrow are released
2) Platelets adhere to fat
b. Can cause adult respiratory distress syndrome
5. Foreign matter
a. Enters bloodstream through trauma, intravenous or intra-arterial lines, or drug particulate, including illicit drug use
b. Coagulation cascade is initiated
c. Thromboemboli form around the particle; ischemia can occur (McCance & Huether, 1998)
C. Effects of PE
1. Produces area of lung that is ventilated but underperfused (ischemia and organic dysfunction)
a. Increased physiologic dead-space ventilation
b. Reflex bronchospasm in affected area as a result of histamine or serotonin release from the clot
1) Thought to be compensatory in the occluded area since it reduces the unevenness of ventilation and perfusion
2) In adjacent lung tissue, bronchospasm may result in considerable hypoxemia
2. Pulmonary hypertension results from large area of reduced flow through pulmonary vascular bed
a. Two thirds of vascular bed must be obliterated prior to development of pulmonary hypertension (Abrams, 1997)
b. One large embolus or multiple recurrent emboli (showers)
3. Infarction
a. Localized area of ischemic necrosis of lung tissue
b. Uncommon because of lung’s dual blood supply (pulmonary and bronchial)
c. Associated with occlusion of medium-size lobar or lobular artery and insufficient collateral flow from the bronchial circulation
d. Pleural friction rub and small pleural effusion are signs (Abrams, 1997)
4. Pain: secondary to ischemia
D. Size of Embolus Determines Outcome/Symptoms
1. Small embolus in patient with healthy lung may be asymptomatic or very subtle/nonspecific
2. Small embolus in patient with lung disease (chronic obstructive pulmonary disease, cancer) may cause severe hypoxia
3. Multiple small emboli (emboli shower) may have similar effect on lung function, oxygenation as one large embolus
4. Massive PE: ‘‘Saddle’’ embolus
a. Complete occlusion of bifurcation of main pulmonary artery
b. Complete obstruction of outflow from right ventricle
c. Sudden shock with severe chest pain, tachycardia, hypotension, cyanosis, stupor, syncope
d. Death within 30 minutes before medical intervention can be initiated
 III. Etiology/Precipitating Factors
III. Etiology/Precipitating Factors
A. All Risk Factors for development of DVT (Virchow triad) as listed in Chapter 19, Superficial Thrombophlebitis and Deep Vein Thrombosis
1. Venous Stasis
a. Age: risk progressively increases over 40; threefold increase in risk after age 70 (Caprini, Arcelus, Hasty, Tamhane, & Fabrega, 1991)
b. Immobilization: bed rest, travel, sedentary work
c. All surgery, especially orthopedic
d. Obesity: increased intra-abdominal pressure plus relative sedentary lifestyle
e. Pregnancy and postpartum state, estrogen therapy: hormonal changes affect vessel wall
f. Acute CVA
g. Spinal cord injury
2. Vessel wall injury
a. Postsurgical or trauma patient: especially chest, abdomen, pelvic, or leg injury
b. Pelvic or hip fractures
c. Intravenous therapy, central venous catheters
3. Hypercoagulability
a. Malignancy
b. Pregnancy (hormone fluctuation)
c. Polycythemia: associated with other pulmonary conditions or congenital heart defects
d. Genetic disorders of coagulability (refer to Chapter 19, Superficial Thrombophlebitis and Deep Vein Thrombosis)
e. Tobacco use
f. Myocardial infarction
g. Nephrotic syndrome
h. Dehydration
i. Inflammatory bowel disease
j. Buerger disease
k. Lupus anticoagulant/anticardiolipin antibody
l. Disseminated intravascular coagulation (DIC)
m. Medications: chemotherapy, estrogen/oral contraception
B. High-Risk Disorders
1. Congestive heart failure is single most important condition predisposing to DVT/PE (Abrams, 1997)
2. Postoperative state
a. Hip surgery
b. Extensive abdominal or pelvic surgery for malignancy
3. Postpartum
4. History of DVT or PE
5. Varicose veins
6. Long bone fractures
7. Abdominal infection
8. Diabetes mellitus
9. Sickle cell anemia
10. Chronic lung disease
11. Malignancy: tumors secrete clotting factor
C. Prevention of PE in surgical and trauma patients (refer to Table 19-1 in Chapter 19, Superficial Thrombophlebitis and Deep Vein Thrombosis)
 IV. Assessment
IV. Assessment
A. Risk Factors
1. Family history of thromboembolism
2. Assess for conditions predisposing to venous thrombosis as listed above
B. Primary Prevention
1. Smoking cessation
2. Public education
a. Awareness of risk factors
b. Promotion of a healthy lifestyle: maintain appropriate weight, exercise/mobility
c. Smoking cessation
d. Control hypertension
3. Assess for presence of risk factors
4. Familial coagulation studies if positive family history
a. Factor V Leiden
b. Protein C
c. Protein S
d. Prothrombin 20210 gene mutation
e. Activated protein C resistance
f. Homocysteine
g. Nonpharmacologic modalities
1) Exercise: “ambulate or elevate!”, ankle rotation, plantar flexion/extension increases venous return
2) Graduated compression stockings for patients with history of DVT, venous insufficiency or ulcer, or varicose veins
a) 20 to 30 mm Hg compression if no prior history of DVT and normal vascular studies
b) 30 to 40 mm Hg compression with positive history of DVT, ulcer, abnormal vascular examination/studies
5. Prevent VTE during air travel: Drink lots of water, move around, avoid alcohol, wear compression hose (Goldhaber & Morrison, 2002)
C. Prevention for hospitalized general medical patients with one or more risk factors
1. Ambulate/exercise as able or appropriate
2. Graduated compression support stocking (18 to 20 mm Hg) if patient has normal arterial examination
3. Weight-based unfractionated heparin or low-molecular-weight heparin (LMWH)
4. PE prevention for surgical and trauma patients (refer to Table 19-1 in Chapter 19, Superficial Thrombophlebitis and Deep Vein Thrombosis)
D. Patient History
1. Signs and symptoms variable, subtle, and nonspecific
a. General health state of patient: small PE may cause significant distress if patient has underlying cardiovascular or pulmonary disease; other patients may be asymptomatic if relatively healthy
b. Size of embolus/occlusion: massive PE may lead to acute right heart failure and cardiopulmonary arrest and death
c. Sudden unexplained hypotension, chest pain, or respiratory distress suggests possibility of pulmonary embolism (Wheeler & Anderson, 1996)
d. Diagnosis of PE often unsuspected: two thirds of deaths from PE occur within 1 hour of embolism and are undiagnosed (Cardin & Marinelli, 2004)
2. Classic subjective symptoms in order of frequency (Thompson & Hales, 2004)
a. Dyspnea: 73%
b. Pleuritic chest pain, especially exacerbated by movement or breathing: 66%
c. Cough: 37%
d. Hemoptysis: 13%
e. Restlessness/anxiety
f. Lightheadedness/fainting
g. Increased respiratory and heart rate
h. Sweating (Goldhaber & Morrison, 2002)
3. Objective patient history
a. Documented history of a pulmonary embolism
b. Recent history of a DVT
c. Family history of thrombophilia
E. Physical Examination/Objective Findings
1. Most common signs, in order of frequency (Thompson & Hales, 2004)
a. Tachypnea/hypoxemia: 70%; caused by mismatch of alveolar ventilation without pulmonary flow in that area
b. Rales: 51%
c. Tachycardia: 30%
d. Fourth heart sound: 24%
e. Accentuated pulmonic component of the second heart sound: 23%
f. Frequency of these findings was the same for patients without PE: no particular clinical finding is sensitive or specific enough to establish diagnosis
2. Inspection
a. Neuro: anxiety, level of consciousness/faint or syncope
b. Skin: diaphoretic, flushed or pale evidence of shock; cyanosis
c. Respiratory: labored breathing, orthopnea, cough/hemoptysis, acute cor pulmonale
d. Cardiovascular: distended neck veins
e. Extremities: peripheral edema (especially unilateral), bulging varicosities, erythema
3. Palpation
a. Pulses usually normal unless edema diminishes ability to palpate or patient in shock
b. Presence and amount of edema
c. Skin temperature/low-grade fever, diaphoresis
4. Percussion
a. Chest: areas of dullness indicate consolidation/fluid collection
b. Abdomen: assess for masses
5. Auscultation
a. Vital signs
b. Tachycardia
c. Tachyon
d. Hypotension/shock
e. Cardiac: murmur, rub, arrhythmia, bruits
f. Lung sounds: rales, pleural friction rub, diminished lung sounds
g. Abdomen: bowel sounds will be diminished if in shock
6. Shock
a. Indicates massive PE in healthy patient or submassive PE in patient with pre-existing cardiopulmonary disease
b. Associated with fivefold increase in mortality (Cardin & Marinelli, 2004)
c. Mechanism: blockage of pulmonary outflow tract by emboli, resulting in severe right ventricular hypertension and failure
F. Considerations Across the Life Span for Increased Risk
1. Young adulthood: contact sports; oral contraception; alcohol, tobacco, and illicit drug use; compliance; presence of other diseases (e.g., diabetes, cancer); nutritional status; other medications (interactions with anticoagulants)
2. Women of childbearing age: oral contraception; planned conception (warfarin crosses placenta and causes birth defects); alcohol, tobacco, and illicit drug use; compliance; other medications such as steroid therapy; varicose veins
3. Elderly: hormone replacement therapy; safety issues: increased risk of falls, home environment, support systems, memory loss, increased bleeding risk; general health/nutritional status (may require a lower dose); use of alcohol, tobacco, and illicit drugs; polypharmacy/medications; prior episodes of DVT/PE; stroke/paralysis; surgery/trauma
 V. Pertinent Diagnostic Testing
V. Pertinent Diagnostic Testing
A. Critical Bedside Laboratory Tests Used for Rapid Diagnosis
1. Chest x-ray after injection of contrast (American Thoracic Society, 2010): 80% are abnormal but nonspecific in majority of patients
a. Important in identifying other cardiac or pulmonary problems which may resemble PE
b. Pulmonary infarct shows triangular, wedge-shaped defect
c. Lung markings (primarily blood vessels) are diminished in area supplied by clotted artery
d. Right heart enlargement
e. Atelectasis, pleural effusion, pulmonary infiltrates, elevated hemidiaphragm may be seen
2. Electrocardiogram (ECG)
a. May show nonspecific changes in T-wave, S-T segment, or axis deviation: if present, may indicate right heart strain secondary to pulmonary arterial obstruction
b. Useful to evaluate for myocardial infarction, arrhythmia, or other cardiac causes of symptoms
3. Arterial blood gases (ABG)
a. Low arterial oxygen (PO2) highly suspicious for PE, especially if chest x-ray normal
b. Usually show hypoxemia, hypocapnia, respiratory alkalosis, but not always seen, so ABGs do not play major role in diagnosis
c. Normal does not exclude PE but makes diagnosis less likely
d. Pulse oximetry is of little diagnostic value, but if <95% at time of diagnosis, patient is at increased risk of complications (e.g., respiratory failure, cardiogenic shock, and death)
4. Noninvasive venous studies (venous duplex ultrasound or impedance plethysmography)
a. Positive study for DVT very helpful in establishing diagnosis and origin or PE
b. Eliminates need for transport for invasive testing if positive
5. D-dimer assay (Thompson & Hales, 2004)
a. A degradation product of fibrin, which is broken down by natural fibrinolysis
b. Quantitative indicator for active clotting process; elevated eightfold after DVT/PE, but also may be increased in presence of infection, cancer, surgery, heart failure, kidney failure
c. Negative (<500 ng/mL) essentially excludes PE in healthy patients with low clinical suspicion and nondiagnostic ventilation/perfusion (V/Q) scan
d. Positive (>500 ng/mL) does not provide specific diagnostic information, especially if there is history of malignancy, recent trauma, or surgery within past 3 months: lacks specificity, especially in advanced age (Thompson & Hales, 2004)
e. Sensitivity and negative predictive value is high: 85% using SimpliRED test (rapid RBC agglutination testing used at bedside). This increased to 99% if patients had low probability of PE from clinical assessment (Thompson & Hales, 2004)
f. Reliance on D-dimer for PE diagnosis is limited
1) Test methodology and assays vary
2) D-dimer analyses using enzyme-linked immunosorbent assays (ELISA) yield best sensitivities (95%) for excluding PE/DVT
3) A negative rapid ELISA is as useful as normal lung scan and negative venous duplex scan to exclude PE
6. Nuclear medicine lung scan (American Thoracic Society, 2010)
a. Shows where blood does not flow
b. Good for seeing smaller lung arteries
B. Noninvasive Testing
1. V/Q scan
a. Assesses airflow and pulmonary circulation to identify areas that may have normal air exchange but abnormal blood flow indicative of obstruction
b. Ventilation scan requires inhalation of radioactive gases or aerosols followed by imaging to detect nonventilated area
c. Perfusion scan requires injection of radioactive material (albumin with technetium or iodine) followed by imaging to detect area of nonperfusion suggestive of blocked artery
d. Pre-existing lung disease (atelectasis, pneumothorax, emphysema, chronic pulmonary lung disease) may cause false-positive results: need to compare chest x-ray and patient history
e. A normal ventilation scan with abnormal perfusion scan is associated with high probability of PE
f. When clinical suspicion for PE is high, and V/Q scan read as high probability for PE, accuracy is 96% (Wheeler & Anderson, 1996)
g. When clinical suspicion low and V/Q is normal, likelihood of PE very low (2% to 9%)
h. Many patients have indeterminate results: further evaluation with invasive studies needed
2. Spiral computed tomography (CT): test of choice in many institutions
a. Employs continuous movement of patient through scanner with concurrent rotating rapid scanning
b. More accurate when intravenous contrast material used
c. Detection of proximal vessel emboli easier than in segmental arteries; limited ability to detect emboli beyond segmental arteries
3. MRI: has been used to make diagnosis but not cost effective or safe in critically ill patients; respiratory and cardiac artifact limit imaging
4. Echocardiography (ECHO)
a. Evaluates for acute cor pulmonale
b. When PE causes >50% pulmonary artery obstruction, severe pulmonary artery hypertension leads to distention of right ventricle and severe right heart failure
c. 80% of patients with known PE have abnormal ECHO
d. ECHO has been used to monitor improvement of obstruction, especially during treatment with fibrinolytic agents
C. Invasive Testing: pulmonary angiography
1. Gold standard for diagnosis of PE, but not often performed (see Limitations)
2. Allows visualization of pulmonary arteries via catheterization of groin, injection of contrast material, and x-ray
3. Internal filling defect in pulmonary artery confirms diagnosis
4. Limitations
a. Invasive procedure using contrast material: need to note allergies
b. Critically ill patient needs to be monitored: may be difficult and dangerous
c. Especially risky for patients with right heart failure, severe pulmonary hypertension, and respiratory failure; mortality from procedure <2%
d. High level of technical expertise; expensive
e. Not suitable for routine use or screening
D. Other Testing: Consider screening for familial/genetic hypercoagulabilities as discussed in Chapter 19 (Superficial Thrombophlebitis and Deep Vein Thrombosis)
 VI. Medical Management of Pulmonary Embolism
VI. Medical Management of Pulmonary Embolism
A. Treatment Goals
1. Maintain adequate cardiac and respiratory function until blockage resolves, usually within 10 to 14 days
2. Prevent recurrence of thrombosis or other embolic events
B. Prompt, Accurate Diagnosis with immediate stabilization using supportive care and anticoagulation therapy required for maximal outcome
1. Elevate head of bed >30 degrees
2. Oxygen via nasal cannula, mask, or both
3. Intubation and mechanical ventilation if needed for severe respiratory distress
4. Intravenous access: large bore needle if possible
5. Small doses of opiates (intravenous morphine, 1 to 2 mg) for discomfort and anxiety; avoid larger doses which may lead to respiratory depression
6. Cautious administration of intravenous fluids to avoid exacerbation of right heart failure
7. Graduated compression stockings
8. Medications to control/stabilize blood pressure, manage dysrhythmias, maintain normal volume status
C. Baseline Laboratory Values Prior to Initiation of Treatment
1. Activated partial thromboplastin time (aPTT), prothrombin time (PT) with international normalization ratio (INR), complete blood count (CBC), creatinine
2. Special coagulation studies, if necessary
3. ABG
4. Stool for occult blood
D. Heparin
1. Cornerstone of treatment for PE (Wheeler & Anderson, 1996)
a. Stops progression of thrombosis
b. Associated with less than 5% recurrence rate of PE
c. Emboli dissolve over course of several days because of natural fibrinolytic mechanisms
d. In cases of incomplete lysis, chronic pulmonary hypertension may result
2. Intravenous unfractionated heparin standard treatment
a. Important to reach therapeutic level as quickly as possible to prevent reoccurrence of PE: 1.5 to 2.5 times baseline aPTT
1) Initial bolus of 120 to 140 units/kg: 5,000 to 10,000 units
2) Infusion of 20 units/kg/hr: usually 1,000 to 2,000 units/hr
3) Check aPTT every 6 hours until stable, adjust heparin as necessary (Feied & Handler, 2000)
4) Close monitoring for first 24 to 72 hours required to prevent under- or over-anticoagulation. “The most common mistake that occurs is to give too little heparin initially when the heparin requirement is high, and too much heparin later, when the heparin requirement is generally low” (Walsh & Rice, 2004).
3. LMWH
a. “With proper dosing, several LMWH products have been found safer and more effective than unfractionated heparin both for prophylaxis and for treatment of DVT and PE” (Feied & Handler, 2000)
b. If creatinine is >2.0 or the platelet count is <100,000, alert the medical provider to discuss use of unfractionated heparin
c. No need to check aPTT, as there is no correlation with LMWH
E. Fondaparinux (Arixtra): factor Xa inhibitor, FDA approved 2001
1. Action
a. Selectively catalyzes factor Xa by antithrombin III without inhibiting thrombin
b. Does not bind to platelet factor 4; does not affect platelet aggregation
2. Current use
a. Prophylaxis of DVT that could lead to PE in patients undergoing hip fracture repair or hip or knee replacement surgery or abdominal surgery (PEGASUS Trial Study, 2005)
b. Dosage for DVT prevention hip and knee replacement: 2.5 mg subcutaneous injection daily for 5 to 9 days, up to 11 days
c. Dosage for prevention of DVT hip fracture surgery: 2.5 mg subcutaneous injection daily for 29 to 33 days
d. Dosage for prevention of DVT in abdominal surgery: 2.5 mg subcutaneous injection 6 hours after surgery for 5 to 9 days
3. Use in PE (The Matisse Investigators, 2003)
a. Fondaparinux and enoxaparin have similar safety and efficacy profile in treatment of PE
b. Dosage for DVT/PE treatment: 5 mg (patient weight <50 kg); 7.5 mg (50 to 100 kg); 10 mg (>100 kg) for 5 to 9 days
c. Continue treatment until therapeutic oral anticoagulation is complete
4. Potential advantages
a. Fewer bleeding complications
b. Minimization/elimination of biological variability, immunogenic reactivity, and pathogenic contamination (less potential for hypersensitivity reactions)
c. Shown to be as safe and effective as LMWH (Buller et al., 2004)
d. Cost: $18.09 for 2.5 mg dose
e. Once-daily dosing; dose does not need to be calculated
5. Disadvantages/adverse reaction
a. Not recommended for patients with severe renal insufficiency, low body weight, active major bleeding, bacterial endocarditis, or thrombocytopenia
b. Common injection site bleeding, rash, pruritus, fever
c. Serious major bleeding, thrombocytopenia, aminotransferase elevation, anemia
d. Drug interactions: many; avoid other anticoagulants, vitamin A, herbal products such as ginkgo, green tea, devil’s claw, St. John’s wort
F. Warfarin Oral Anticoagulation
1. Initiated on day 1 of heparin therapy if possible, usually 5 to 15 mg
2. Adjust subsequent daily dose according to the INR, with goal of 2 to 3
3. Overlap heparin and warfarin for 5 to 7 days or until INR stable at therapeutic goal for 48 hours to allow for depletion of vitamin K-dependent clotting factors
4. Anticoagulate with warfarin for 3 to 6 months (Feied & Handler, 2000)
5. If the individual has a familial or acquired thrombophilia, recurrent thromboembolism or ongoing risk factors, anticoagulation may be lifelong (refer to Chapter 19, Superficial Thrombophlebitis and Deep Vein Thrombosis for chronic anticoagulation management)
G. Fibrinolytic Therapy
1. Dissolution of clot via chemical lysis of fibrin component, resulting in rapid removal of intraluminal thrombus and restoration of vessel patency
2. Potential benefits
a. Prompt dissolution of physiologically compromising occlusion of pulmonary circulation: may be life-saving; clot lysis usually occurs in 80% to 90% of cases
b. Faster recovery
c. Prevention of recurrent thrombus formation
d. Rapid restoration of hemodynamic function
e. Compared to use of conventional heparin alone, improved long-term right ventricular function and less pulmonary hypertension (Wheeler & Anderson, 1996)
3. Disadvantages
a. High risk of systemic bleeding complications: double or triple compared to heparin (Erdman, Rodvold, & Friedenberg, 1997)
b. Most serious is intracranial bleeding: 1% incidence (Wheeler & Anderson, 1996)
c. Intraperitoneal bleeding may be life threatening
d. Close monitoring: intensive care if patient unstable
e. Cost of medication
f. Studies have not shown improved overall mortality compared to heparin (Erdman et al., 1997)
4. Proposed indications
a. Massive/submassive PE with hemodynamic compromise
b. Massive PE without hemodynamic compromise
c. Submassive PE in patients who cannot tolerate further cardiopulmonary compromise
d. Heparin treatment failures
e. Extensive proximal DVT
5. Contraindications to thrombolytic therapy (Erdman et al., 1997)
a. Absolute: active bleeding, cerebrovascular disease/event or procedure within past 2 months
b. Relative: recent major surgery or trauma (within 10 days), postpartum, cardiopulmonary resuscitation with rib fractures, thoracentesis, paracentesis, lumbar puncture, any condition requiring maintenance of normal clotting or healing
c. Potentially serious bleeding: uncontrolled coagulation defects, severe hypertension, pregnancy, any condition with potential bleeding risk
6. FDA approved fibrinolytic agents
a. Streptokinase: first agent approved but is no longer manufactured
b. Urokinase: urokinase is not currently available since Abbokinase is no longer manufactured and Kinlytic is not yet FDA approved
c. Recombinant tissue plasminogen activator (rt-PA)
1) 100 mg as continuous infusion over 2 hours
2) More rapid and complete thrombus dissolution compared to urokinase
3) Shortest treatment time
4) Alteplase is only FDA approved for pulmonary embolism (Jaff et al., 2011)
7. Precautions and guidelines for patient care
a. Minimizing bleeding
1) Careful patient selection/evaluation with attention to neurologic history
2) Laboratory monitoring prior to infusion: CBC, platelet count, aPTT, and PT, and tests for coagulation defects (e.g., fibrin degradation products); repeat as indicated during and after therapy
3) Fecal occult blood sample prior to therapy
4) Minimal venipuncture and arterial puncture
5) Short infusions via pulmonary artery catheter (usually inserted in groin) directed into thrombus; adequate pressure on site once catheter removed
6) If possible, avoidance of lytic agents as emergency treatment: can be used successfully as an elective procedure via peripheral vein with less bleeding risk (Wheeler & Anderson, 1996)
7) Avoid administration of heparin: if heparin recently given, wait until aPPT is less than 1.5 control. Once lytic therapy complete, do not resume heparin until aPPT <80 seconds (Walsh & Rice, 2004)
b. Monitor for adverse effects
1) Hemorrhage: major bleeding occurs in approximately 20% (Erdman et al., 1997). Lytic agents have short half-life: discontinuation results in rapid cessation of lytic activity. If blood replacement required, whole blood, packed red cells, fresh-frozen plasma, or cryoprecipitate may be given. If bleeding continues, 5 g doses of E-aminocaproic acid (EACA) may be given
2) Allergic reaction: mostly associated with streptokinase, although mild allergic reactions to other agents have been reported; urticaria, itching, flushing, nausea, headache, alteration of blood pressure. Treat with antihistamines
3) Fever: seldom greater than 104°F; 30% with streptokinase; 15% with other agents. Treat with acetaminophen
4) Hypotension: associated with rapid or high-dose infusions of any lytic agents; treat by slowing infusion
5) Thrombocytopenia: reported in 10% of rt-PA infusions; discontinue if platelet count falls below 75,000/mm3
c. Guidelines for patient care (Erdman et al., 1997)
1) Minimal repositioning of patient
2) Discontinue parenteral medications
3) Substitute oral medications when possible
4) Avoid invasive procedures
5) Apply compression at venipuncture sites
6) Avoid any other anticoagulants
7) Avoid use of platelet-active drugs, aspirin-containing drugs, antiplatelet agents, and dextran
 VII. Surgical Management
VII. Surgical Management
A. Inferior Vena Cava Filter Placement
1. Indications (see Table 20-1)
2. Percutaneous insertion technique in radiology (Feied & Handler, 2000)
a. Local anesthetic and small incision in groin
b. Catheter is introduced into the femoral vein
c. Radiopaque contrast is administered and filter is placed in the inferior vena cava, with apex near the level of the lowest renal vein at approximately L2 and L3
d. Requires anticoagulation with heparin and followed by lifelong warfarin
3. Devices
a. Permanent
1) Greenfield filter: either stainless steel or titanium; only device shown to maintain patency of inferior vena cava and avoid renal vein occlusion (Greenfield & Proctor, 1996)
2) Nitinol filter
3) Bird’s nest filter
4) Vena Tech
5) TrapEase
6) SiteFlo (Siskin & Cho, 2011).
b. Retrievable filter: may be removed between 14 and 41 days of placement or remain in place as permanent filter; reported technical success rate of 93% (Morris, Rogers, Najarian, Bhave, & Shackford, 2004)
1) ALN
2) G2
3) Eclipse
4) Gunther-Tulip
5) Celect
6) OptEase
7) Option (Siskin & Cho, 2011)
TABLE 20-1 Indications for Insertion of a Vena Cava Filter
| Deep Vein Thrombosis (DVT) or Pulmonary Embolism (PE) in patients with contraindication or complication to anticoagulants |
| Prevention of PE in patients undergoing surgery, cancer, or trauma |
| Failure of anticoagulants |
| IVC or iliofemoral thrombus |
Siskin, G., & Cho, K. (2011). Inferior vena cava filters. Retrieved from http://emedicine.Medscape.com/article/419796-overview, accessed November 30, 2012.
4. Potential complications (Walsh & Rice, 2004)
a. Recurrent PE
b. Venous insufficiency
c. Air embolism
d. Occlusion of device with bilateral venous outflow obstruction
e. Improper placement or migration
f. Hematoma at the insertion site
B. Pulmonary Embolectomy
1. Indications: usually only for life-threatening situations, although some centers suggest improved surgical technique and careful patient selection may expand use of this procedure (Aklog, Williams, Byrne, & Goldhaber, 2002)
a. Massive PE; patient pre-terminal
b. Thrombolytic therapy unavailable or contraindicated
c. Most patients die before they can be transported to operating room or stabilize and no longer require procedure
d. Rarely used electively for patients with chronic PE where clot did not naturally lyse
2. Procedure
a. Balloon-tipped catheter is inserted into the pulmonary artery via incision in groin
b. The clot is removed by withdrawal of the inflated catheter
c. Immediately followed by vena caval filter placement and anticoagulation
d. Rheolytic thrombectomy: high-velocity saline solution to create a strong Venturi effect, followed by aspiration of the clot with catheter or syringe
e. Open surgical embolectomy: used for patients with adequate systolic arterial pressure but profound right ventricular failure; beating heart bypass technique (Aklog et al., 2002; Cardin & Marinelli, 2004)
 VIII. Nursing Diagnosis
VIII. Nursing Diagnosis
A. Alteration in tissue perfusion related to pulmonary arterial obstruction as evidenced by dyspnea, tachypnea, and tachycardia
1. Expected patient outcomes: patient will maintain end-organ perfusion
2. Interventions
a. Supplemental oxygen and/or mechanical ventilation as indicated
b. Elevate head of bed; unaffected lung positioned down to allow for increased blood flow to area of continuous perfusion/ventilation (e.g., if PE in left lung, patient lies on right side)
c. Assist patient with activities of daily living
d. Turn, cough, and deep breathe
e. Hydrate patient cautiously, avoiding overhydration and exacerbation of right heart failure
3. Evaluation
a. Monitor oxygen saturation either continuously or with each assessment and p.r.n.
b. Auscultate lung and heart sounds with each assessment and p.r.n.
c. Check vital signs as frequently as level of acuity indicates is appropriate
d. Observe for dyspnea, tachycardia, diaphoresis, anxiety, or other symptoms of distress with any activity
B. Alteration in comfort related to obstruction of pulmonary circulation
1. Expected patient outcomes
a. Patient will be pain free
b. Patient will be able to perform activities of daily living
2. Interventions
a. Position of comfort and optimal oxygenation (good lung down)
b. Analgesia p.r.n.
c. Support chest with a pillow when coughing
d. Activity as tolerated
3. Evaluation
a. Assess pain on a scale of 1 to 10 minimally every 8 hours and p.r.n.
b. Observe/record frequency of requests for pain medication
c. Observe tolerance of activity: increased pain, tachycardia, hypertension
d. Observe/record response to analgesia
C. Anxiety related to dyspnea, pain, and unknown treatments/diagnostics
1. Expected outcomes
a. Patient will appear physically calm/comfortable and express feeling less anxious
b. Patient will verbalize understanding of related tests and medical/nursing treatments
2. Interventions
a. Administer medication as indicated for control of anxiety in acute stage of illness
b. Provide patient/family education regarding disease process, treatment, diagnostics, and expected outcomes
c. Provide calm atmosphere
d. Use open-ended questions with patient
e. Allow patient and family to verbalize feelings
3. Evaluation
a. Assess for physical signs of anxiety hourly and p.r.n. during acute illness; then every 8 hours once stable
b. Assess feelings as patient verbalizes emotions
c. Note patient’s interactions with others
 IX. Patient Teaching
IX. Patient Teaching
A. Disease Prevention/Health Promotion and Secondary Prevention
1. Define PE to patient/family and explain why this occurred, with discussion of specific situation if known (e.g., thrombus formation associated with recent air travel)
2. Identify patient’s risk factors
3. Explain treatment goals
a. Prevention of subsequent thrombosis/embolism
b. Maintenance of activities of daily living or pre-illness level of function
c. Avoidance of complications of therapy
d. INR goal: define and give expected range (usual 2 to 3)
4. Anticoagulant medication education
a. Tablet strength, color, how many, and how often to take
b. Side effects
1) Easy bruisability
2) Medical attention for new rash or significant bleeding
c. Interactions with other medications
1) No ASA unless prescribed by care provider
2) Multiple drug and food interactions with warfarin
3) Discuss any new OTC or prescription products prior to starting
d. Subcutaneous injection technique if self-administering heparin or LMWH
e. Discuss estrogen therapy risks with medical provider
5. Symptoms to watch for and what to do
a. Infection: call medical provider
b. Increased pain, swelling, warmth, erythema of leg: seek emergency care, call 911
c. Chest pain, rapid pulse, sweating, anxiety, fainting, hemoptysis: seek emergency care, call 911
d. Unilateral paralysis, numbness, facial droop, vision or speech change, loss of coordination, change in mental status: seek emergency care, call 911
e. Bleeding
1) Major: call 911 or go to emergency room
2) Minor: alert health care provider
B. Home Care Considerations
1. Diet
a. Vitamin K: can have a negative effect on warfarin
1) Diet should be consistent and moderate
2) Give list of foods that are moderate to high in vitamin K
a) Green leafy vegetables are high in vitamin K
b) Green tea and tobacco are very high in vitamin K and should be avoided
b. Avoid alcohol use
c. Maintain desired weight (BMI between 19 and 25)
d. Check with health care provider before using any herbal remedies
2. Activity
a. Walk regularly/daily: may also jog, swim, cycle
b. Avoid prolonged sitting or standing
c. Elevate legs when nonambulatory
d. Avoid crossing legs
3. Avoid constrictive clothing and footwear: compression support hose should not be overly binding and is best obtained from experienced compression garment fitter
4. Safety precautions
a. Protect the feet
b. Exercise caution when using sharp items such as scissors, broken glass, and so forth
c. Wear a helmet when riding a bicycle
d. Wear or carry identification that indicates anticoagulation therapy
e. Seek immediate care for serious injury or head injury
5. Home environment and support system concerns
a. Ability to come in for routine laboratory/INR
b. Ability to administer own medications accurately
c. Review fall risks and recommend necessary changes or precautions
6. Call nurse or medical provider for
a. Changes in medication, diet, missed dose
b. Questions or symptoms they have
 X. Research and Further Study (American Thrombosis Society, 2010)
X. Research and Further Study (American Thrombosis Society, 2010)
A. Genetic Factors: link being discovered between genetics and blood clotting problems
B. Information on Blood Clotting Process: improving
C. Improved Diagnostic Tools
D. New Anticoagulant Agents: options that can be used when main frontline agents are contraindicated
CASE STUDY
J.D. is a 54-year-old tree service owner who suffered a back injury after falling out of a tree. He did not seek immediate medical attention but placed himself on bed rest for 5 days and took acetaminophen 1,000 mg three times daily for pain. Mr. D. subsequently presented to the emergency room with chest pain and dyspnea. He is on no other medications and denied any other health issues. He specifically denied any bleeding problems. Immediate examination upon presentation includes the following:
Integument: diaphoretic; otherwise, skin intact without bruises or rash
Cardiovascular: apical pulse 120, regular without murmurs, S1, S2. B/P 130/70; uniform mild edema of right lower extremity from proximal thigh to ankle, radial and pedal pulses +4
Respiratory: rate 24, even and labored; chest clear bilaterally with no areas of dullness to percussion
Gastrointestinal: bowel sounds present in all four quadrants; no organomegaly, bruits, or masses
Neurologic: no deficits; alert, oriented, and appropriate but appears anxious with rapid speech and restlessness
Social history: independent ADLs; lives with his wife in a one-story home; two daughters who live close; smokes 1 pack per day for past 40 years; drinks 1 to 3 beers after work daily
Family history: mother, age 81, and two maternal uncles, both deceased, have history of DVT
Laboratory values reveal the following:
Hgb: 14 (normal)
Platelets: 180,000 (normal)
Creatinine: 1.0 (normal)
ABGs SaO2: 88 (abnormal)
ECG tachycardia with nonspecific T-wave and ST segment changes
Initial diagnosis: pulmonary embolism and right lower extremity DVT suspected
Review Questions
1. What signs and symptoms are suspicious for thromboembolism?
a. Dyspnea with tachypnea and labored respirations
b. Chest pain
c. Tachycardia
d. Anxiety
e. All of the above
2. What diagnostic test is most helpful in establishing the diagnosis of PE?
a. Electrocardiogram
b. Arterial blood gas
c. Chest x-ray
d. Venous duplex ultrasound
e. D-dimer assay
3. Mr. D. is sent for a spiral CT scan to confirm the diagnosis of PE, rather than a ventilation/perfusion (V/Q) scan. What conditions might lead to an erroneous V/Q scan result?
a. Chronic obstructive pulmonary disease (COPD)
b. Myocardial infarction
c. Atherosclerosis
d. Spinal stenosis
4. What immediate care measures are essential in ensuring Mr. D’s safety and maintenance of normal gas exchange?
a. Trendelenburg position
b. Administration of oxygen and 1 mg of morphine sulfate
c. 1 L of normal saline rapidly infused over 30 minutes
d. Graduated compression stockings
5. In treating Mr. D’s pulmonary embolism, the most common mistake is to give too little anticoagulation initially when the heparin requirement is high, and too much heparin later, when the heparin requirement is lower. True or False?
6. What laboratory tests are recommended prior to initiation of heparin therapy?
a. CBC with platelet count
b. Creatinine
c. Stool for occult blood
d. Familial coagulopathy tests
e. All of the above
7. Patient and family teaching at the time of Mr. D’s discharge should emphasize:
a. Avoidance of all green leafy vegetables
b. Smoking cessation and use of a medication bracelet
c. Limited walking for 3 months
d. Discussion of anticoagulation side effects and his work responsibilities
e. a and b
f. b and d
Answers/Rationales
1. e. All of the above. While all of the presenting symptoms are nonspecific and could indicate other disorders (such as myocardial infarction), the most classic subjective symptom is dyspnea, followed by pleuritic chest, cough, hemoptysis, restlessness. Objective findings include tachypnea with hypoxia, tachycardia, rales, and the presence of a fourth heart sound.
2. d. Venous duplex ultrasound. A positive venous ultrasound which demonstrates presence of deep vein thrombosis is very helpful in establishing diagnosis of PE and eliminates need for further invasive testing. The information obtained on other tests may be supportive but is not confirmatory, although a negative D-dimer assay essentially rules out the possibility of a PE in a patient who is otherwise at low risk and has low index of suspicion (which is NOT Mr. D). While pulmonary angiogram remains the gold standard for absolute diagnosis of PE, it is invasive and may be very risky in a critically unstable patient.
3. a. COPD. Mr. D. has a history of smoking for 40 years and likely has either emphysematous changes of his lungs or COPD. Both of these conditions, as well as pneumothorax or atelectasis which could have occurred when he fell out of the tree, can lead to false-positive V/Q results.
4. b. Administration of oxygen and 1 mg of morphine sulfate. He has hypoxemia and is anxious; treating both will stabilize his respiratory and cardiac function, allowing his tachypnea and tachycardia to resolve. Close monitoring for further deterioration is essential: continuous pulse oximetry, cardiac monitoring, cautious administration of fluids to prevent worsening right heart failure but access via a large bore IV in case of rapid decline. Head of bed should be elevated with positioning so that the good (unaffected) lung is down to maximize blood flow to perfused lung tissue. Graduated compression stockings are recommended but are not immediately essential to stabilize the patient.
5. True. Heparin remains the cornerstone of treatment for PE. Early (within first 24 hours) achievement of a therapeutic aPPT (1.5 to 2.5 baseline) has been shown to stop progression of thrombosis and lower recurrence rate. However, continuing high infusion rates beyond 72 hours results in higher risk for bleeding complications.
6. e. All of the above. Mr. D. needs to be assessed for presence of anemia and platelet abnormality prior to anticoagulation to establish a baseline in case he should develop bleeding complications or platelet abnormality as a reaction to heparinization. His anticoagulation therapy would require dose adjustment in the presence of renal insufficiency, and he may not be a candidate for anticoagulation if he has an occult GI bleed. Since his mother and two uncles have a history of venous thrombosis, he (and other family members) should also be assessed for genetic hypercoagulability, although this is not immediately essential since he has several other obvious risk factors for venous thrombosis.
7. b and d. Helping Mr. D understand the risk factors for recurrence of PE can be life saving. They are specifically smoking and his family history, as well as possibility of future work-related trauma. He cannot change his family history, but he needs information regarding important lifestyle changes, including smoking and alcohol cessation, ambulation, leg elevation when not walking, avoidance of trauma, maintenance of desired weight, and a consistent diet. His high-risk occupation should be frankly discussed to investigate possible changes to provide a safer environment. In addition, he needs to be given information on how to contact his health care providers, seek emergency care if needed, and have as complete an understanding of anticoagulation effects and need for follow-up care as possible, with social support systems in place as his circumstances require.
REFERENCES
Abrams, G. D. (1997). Disturbances in circulation. In S. A. Price, & L. M. Anderson (Eds.), Pathophysiology: Clinical concepts of disease process (5th ed., pp. 92–107). St. Louis, MO: Mosby-Year Book.
Aklog, L., Williams, C. S., Byrne, J. G., & Goldhaber, S. Z. (2002). Acute pulmonary embolism: A contemporary approach. Circulation, 105, 1416–1419.
American Thoracic Society. (2010). Chapter 16: Pulmonary embolism in breathing better in America. Retrieved from http://www.thoracic.org/education/breathing-in-america/index.php, accessed November 30, 2012.
Buller, H. R., Davidson, B. L., Decousus, H., Gallus, A., Gent, M., Piovella, F., … Matisse Investigators. (2004). Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: A randomized trial. Annals of Internal Medicine, 140(11), 867–873.
Caprini, J. A., Arcelus, J. I., Hasty, J. H., Tamhane, A. C., & Fabrega, F. (1991). Clinical assessment of venous thromboembolic risk in surgical patients. Seminars in Thrombosis and Hemostasis, 17(Suppl. 3), 304–312.
Cardin, T., & Marinelli, A. (2004). Pulmonary embolism. Critical Care Nurse Quarterly, 27(4), 310–322.
Erdman, S. M., Rodvold, K. A., & Friedenberg, W. R. (1997). Thromboembolic disorders. In J. T. Dipiro, R. L. Talbert, G. C. Yee, G. R. Matzke, B. G. Wells, & L. M. Posey (Eds.), Pharmacotherapy: A pathophysiologic approach (3rd ed., pp. 399–434). Stamford, CT: Appleton & Lange.
Feied, C., & Handler, J. (2000). Pulmonary embolism. Emedicine-Instant Address to the Minds of Medicine. Retrieved from http://www.emedicine.com/EMERG/topic490.html
Goldhaber, S., & Morrison, R. (2002). Pulmonary embolism and deep vein thrombosis. Circulation, 106, 1436–1438.
Greenfield, L. J., & Proctor, M. C. (1996). Diagnosis and management of pulmonary embolism. The Annals of Thoracic Surgery, 61(3), 1037–1038.
Heit, J. A., Silverstien, M. D., Mohr, D. N., Petterson, T. M., Lohse, C. M., O’Fallon, W. M., & Melton, L. J., III. (2001). The epidemiology of venous thromboembolism in the community. Thrombosis and Haemostasis, 86, 452–463.
Jaff, M., McMurtry, M. S., Archer, S. L., Cushman, M., Goldenberg, N., Goldhaber, S. Z., … Zierler, B. K. (2011). Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation, 123, 1788–1830.
McCance, S. L., & Huether, S. E. (1998). Pathophysiology: The biological basis for disease in adults and children (3rd ed., pp. 968–1023). St. Louis, MO: Mosby.
Morris, C. S., Rogers, F. B., Najarian, K. E., Bhave, A. D., & Shackford, S. R. (2004). Current trends in vena caval filtration with the introduction of a retrievable filter at a level I trauma center. Journal of Trauma, 57(1), 32–36.
PEGASUS Trial Study. (2005). Clinical trial results. Retrieved from http://www.trialresultscenter.org/study7632-PEGASUS.htm, accessed August 17, 2013.
Roach, H. D., & Laufman, H. (1955). Relationship between pulmonary embolism and pulmonary infarction: An experimental study. Annals of Surgery, 142(1), 82–91.
Rosendaal, F. R. (1999). Venous thrombosis: A multicausal disease. The Lancet, 353(9159), 1167–1173.
Siskin, G., & Cho, K. (2011). Inferior vena cava filters. Retrieved from http://emedicine.Medscape.com/article/419796-overview, accessed November 30, 2012.
The Matisse Investigators. (2003). Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. New England Journal of Medicine, 349, 1695–1702.
Thompson, B. T., & Hales, C. A. (2004). Clinical manifestations and diagnostic strategies for acute pulmonary embolism. Retrieved from http://www.uptodate.com
Walsh, M. E., & Rice, K. L. (2004). Venous thrombosis and pulmonary embolism. In V. Fahey (Ed.), Vascular nursing (4th ed., pp. 365–397). Philadelphia, PA: W.B. Saunders.
Wheeler, H. B., & Anderson, F. A. (1996). Pulmonary embolism. In P. Gloviczki, & J. S. T. Yao (Eds.), Handbook of venous disorders (pp. 274–291). London, UK: Chapman & Hall.
SUGGESTED READINGS
American Thoracic Society. (1999). The diagnostic approach to acute venous thromboembolism. American Journal of Respiratory and Critical Care Medicine, 160, 1043–1066.
Autar, R. (1996). Nursing assessment of clients at risk of deep vein thrombosis (DVT): The autar DVT scale. Journal of Advanced Nursing, 23(4), 763–770.
Berg, D. E. (1992). Components and defects of the coagulation system. Nurse Practitioner Forum, 3(2), 62–71.
Breen, P. (2000). DVT: What every nurse should know. RN, 63(4), 59–62.
Dolovich, L. R., Ginsberg, J. S., Douketis, J. D., Holbrook, A. M., & Cheah, G. (2000). A meta-analysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism. Archives of Internal Medicine, 160, 181–187.
Geerts, W. H., Heit, J. A., Clagett, G. P., Pineo, G. F., Colwell, C. W., Anderson, F. A., Jr., & Wheeler, H. B. (2001). Prevention of venous thromboembolism. Sixth ACCP Consensus Conference on Antithrombotic Therapy. Chest, 119(1), 132S–175S.
Goldhaber, S., Fanikos. (2004). Prevention of deep vein thrombosis and pulmonary embolism. Circulation, 110, e445–e447.
Grady, D., Wenger, N. K., Herrington, D., Khan, S., Furberg, C., Hunninghake, D., … Hulley, S. (2000). Postmenopausal hormone therapy increases risk for venous thromboembolic disease. Annals of Internal Medicine, 132(9), 689–696.
WEB SITES
Anonymous. (1996). Low-molecular-weight heparin may reduce hospital stays. Mayo clinic health oasis. Retrieved from http://www.mayohealth.org/mayo/9701/htm/heparin.htm
Anonymous. (1998). Pulmonary embolism-what you know may save your life. Mayo clinic health oasis. Retrieved from http://www.mayohealth.org/mayo/9807/htm/pulmonary.htm
Anonymous. (2000). Ask the Mayo physician. Mayo clinic health oasis. Retrieved from http://www.mayohealth.org/mayo/askphys/qa970604.htm
Beer, A. E. Thrombophilia: Inherited and acquired. Reproductive Medicine Program, Finch University of Health Science, Chicago Medical School. Retrieved from http://www.repro-med.net/papers/thromb.html
Beer, A. E., Kwak-Kim, J., Ntrivalas, E., & Chung, H. S. Acquired thrombophilia antiphospholipid antibody syndrome category 2 immune problems. Reproductive Medicine Program, Finch University of Health Science, Chicago Medical School. Retrieved from http://www.repro-med.net/papers/apa.html
Gallus, A. S. (1998). Treatment of deep leg vein thrombosis. Australian Prescriber, 21, 64–66. Retrieved from http://australianprescriber.com/magazines/vol21no3/vein_thrombosis
Kujovich, J., & Goodnight, S. (1999). Factor V Leiden thrombophilia. Gene Clinics, University of Washington, Seattle. Retrieved from wysiwyg://78/http://www.geneclinics.org/profiles/factor-v-leiden/details.html
Peripheral Venous Disorders. Columbia University college of p & s complete home medical guide. Retrieved from http://cpmcnet.columbia.edu/texts/guide/hmg16_0007.html
< div class='tao-gold-member'>



