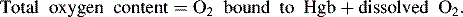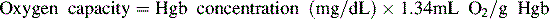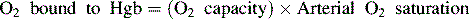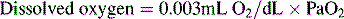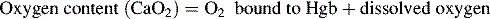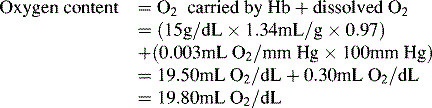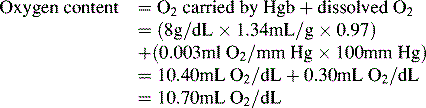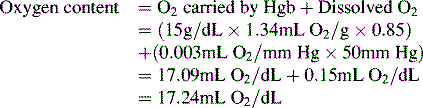9 Pulmonary Disorders
Pearls
• Acute disease of the respiratory tract is the most common cause of illness in infancy and childhood.
• Respiratory disease is frequently present in critically ill or injured children; it may be present as a primary clinical problem or as a secondary complication.
• Altered level of consciousness in an infant or child with respiratory disease is often an ominous sign of deterioration.
• Children can have adequate oxygen saturation as documented by pulse oximetry, but they may be hypoxic because of inadequate oxygen delivery.
• Airway resistance increases exponentially as airway lumen size decreases.
• Once the PaO2 falls below 60 mm Hg, even small additional decreases in PaO2 are associated with a significant fall in arterial oxygen saturation and therefore in arterial oxygen content.
Essential anatomy and physiology
Embryology of the Lung
The respiratory system begins to develop by the fourth week of gestation. A lung bud branches from the primitive esophagus and eventually forms the airways and alveolar spaces. The pulmonary arteries form near the branching airways and their growth matches the growth of the airways. Although virtually all other body systems are physiologically ready for extrauterine life by as early as 25 weeks’ gestation, the lungs require more time to mature. Thus lung maturity is the single most important factor that determines whether a premature infant can survive extrauterine life. Table 9-1 summarizes development of the respiratory system. Although the number of airway branches is fixed at birth, airway dimensions increase until the child is approximately 8 years old.202 Alveoli multiply rapidly from an estimated 20 million alveoli at birth to 200 million by 3 years of age, and the number decreases thereafter. The alveolar surface is lined with type I and type II epithelial cells that are well developed at birth.99
Table 9-1 Fetal Respiratory System Development
| Period of Gestation | Development |
| 26 days | Lower respiratory system begins to develop until separation of the respiratory tract from the foregut is achieved |
| 5 weeks | Lung buds form and begin to differentiate into the bronchi |
| 7-10 weeks | Development of the larynx |
| 5-16 weeks | Twenty-four orders of airway branches are formed |
| 13-25 weeks | Canalicular period; bronchi enlarge and lung tissue becomes highly vascular |
| 26-28 weeks | Lungs are capable of gas exchange; type II alveolar cells secrete surfactant |
| 24 weeks to birth | Capillary network proliferates around the alveoli; approximately 8%-10% of cardiac output flows through the lung; pulmonary vascular resistance is high |
Anatomy of the Chest
The thoracic cavity is formed by the ribs, intercostal muscles, and diaphragm, and it contains both lungs and the mediastinal structures. The right lung is composed of three lobes, and the left lung is composed of two lobes.49 The heart, great vessels, nerves, trachea, and esophagus are located within the mediastinum. Pleural tissue covers each lung and adheres to the surface of the diaphragm and inner surface of the chest wall.
The diaphragm is innervated on each side of the chest by the phrenic nerve, which is formed by the third, fourth, and fifth cervical spinal nerves. In older children and adults, the chest wall is relatively rigid compared with the chest wall of the neonate and infant. Therefore, when the diaphragm contracts in older patients, intrathoracic pressure falls in proportion to the movement of the diaphragm, and air moves into the lungs (Fig. 9-1).
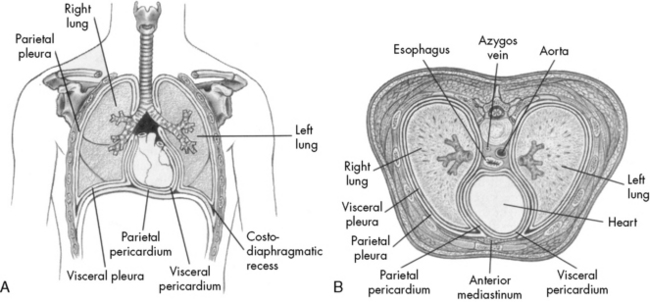
Fig. 9-1 Chest cavity and related structures. A, Anterior view. B, Cross section.
(From Thompson JM, et al: Mosby’s manual of clinical nursing, ed 2, St Louis, 1989, Mosby.)
The ribs angle downward, from back to front, so that contraction of the external intercostal muscles will elevate the rib cage. The chest wall of an infant is compliant, and the external intercostal muscles stabilize the chest wall. When respiratory disease develops, pulmonary compliance is reduced. When the diaphragm contracts and produces a decrease in intrathoracic pressure, intercostal and sternal retractions develop rather than inflation of the lungs (Fig. 9-2). The more the chest wall retracts, the less the lungs inflate.
The diaphragm inserts more horizontally in infants than in older children or adults, and diaphragm contraction can contribute to subcostal retractions, particularly when the infant is supine.49 The greater the retractions present, the more the diaphragm will need to contract or shorten to generate an adequate VT. Retractions make ventilation inefficient, with the result that the diaphragm must shorten and move as much as 130% of normal to generate a VT; this increases the work of breathing and can lead to respiratory muscle fatigue.
The Upper Airway
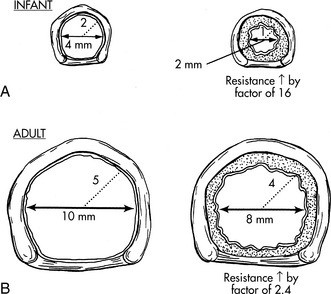
The airways of infants and children are much smaller than the airways of adults. Resistance to air flow in any airway will increase exponentially if the airway radius is compromised (see Box 9-1 and Fig. 9-3). This means that any decrease in airway radius can significantly compromise effective gas flow or increase the work of breathing. Relatively small amounts of mucus accumulation, airway constriction, or edema can substantially reduce airway radius in the infant or child, resulting in an increase in the resistance to air flow and the work of breathing.
Box 9-1 Poiseuille’s Law
R = 8nl/πr4 when flow is laminar (substitute r5 power if flow is turbulent)
l, Length of tube; n, gas viscosity; r, radius of tube.
The infant upper airway is shaped like a funnel, whereas the upper airway of the older child and adult is more tubular. The glottis of an infant is located more anteriorly and more cephalad than in an older child, and the epiglottis is longer, making intubation of the trachea more difficult in the small infant, especially when the neck is hyperextended. The narrowest portion of the infant’s airway is at the level of the cricoid, whereas the narrowest portion of the airway in the adult is at the level of the vocal cords. Small amounts of edema or obstruction in the cricoid (subglottic) area will produce an increase in airway resistance and can lead to respiratory failure. Postnatally the airways increase in both length and diameter and major changes occur in the terminal respiratory units as the number and size of the alveoli increase.49,182
Compliance and Resistance
From the time of the first breath, elastic fibers in the lung tissue create a tendency for the lungs to recoil inward (away from the chest wall). This recoil tendency is balanced by the propensity of the chest wall to spring outward. The net effect of these two opposing forces is to create a subatmospheric pressure in the intrathoracic space at the end of a normal breath (Fig. 9-2). During inspiration, the volume of the thoracic cavity is increased, and intrathoracic pressure becomes more negative with respect to atmospheric pressure. As a result, air moves from the mouth to the alveolar spaces. At the end of inspiration, the elastic recoil of the lungs and chest wall cause alveolar pressure to rise above atmospheric pressure, producing expiratory flow. In a person with normal lungs, expiration is passive and requires no muscular work.92
In addition, resistance to air flow is inversely proportional to the fourth power of the airway radius (Box 9-1) when flow is laminar.110 Any reduction in the infant’s airway radius will result in exponential increases in the resistance to air flow and work of breathing (Fig. 9-3). Resistance to airflow is inversely related to the fifth power of the airway radius when air flow is turbulent (e.g., with upper airway obstruction).
Ventilation
The rate of removal of carbon dioxide from alveoli and the rate of oxygen delivery to the alveoli are directly related to alveolar ventilation. Normal alveolar ventilation is defined as the level of ventilation that results in normal partial pressures of oxygen and carbon dioxide in arterial blood.103
Anatomic dead space is just one part of the total dead space ventilation. A more clinically significant portion of dead space is the physiologic dead space. This space represents the volume of ventilation that reaches the alveoli that do not receive any pulmonary blood flow; therefore it is ventilation that does not participate in gas exchange. Ventilation of this portion of the lung is wasted. This concept is illustrated in Fig. 9-4. Normally, physiologic and anatomic dead space volumes are similar, but physiologic dead space can be significant in patients with pulmonary vascular disease or when positive end-expiratory pressure (PEEP) reduces pulmonary blood flow or whenever right ventricular output is reduced.
Lung Volumes
The total volume of the gas contained in the lung at maximum inspiration is the total lung capacity, Fig. 9-5. The volume that can be expired after a maximal inspiratory effort is the vital capacity (VC). This important and useful measurement of lung function is discussed in detail later in this chapter. VC can be reduced by any acute or chronic lung disease that increases lung stiffness (i.e., reduces lung compliance) or by conditions that limit available intrathoracic space (e.g., scoliosis, pneumonia, pleural effusion).
Ventilation-Perfusion Relationships
Intrapulmonary shunting exists in areas of the lung where alveolar ventilation is absent, but blood flow to the nonventilated alveoli persists (i.e., alveoli are not ventilated but are perfused, so 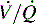 is 0; see Fig. 9-4, C). Intrapulmonary shunting is the cause of reduced PaO2 (hypoxemia) in diseases such as cardiogenic and noncardiogenic pulmonary edema (acute respiratory distress syndrome [ARDS]). By definition, hypoxemia associated with intrapulmonary shunting does not respond to supplementary oxygen administration, because the oxygen does not reach the nonventilated alveoli. This finding is in contrast to hypoxemia caused by partially ventilated alveoli (
is 0; see Fig. 9-4, C). Intrapulmonary shunting is the cause of reduced PaO2 (hypoxemia) in diseases such as cardiogenic and noncardiogenic pulmonary edema (acute respiratory distress syndrome [ARDS]). By definition, hypoxemia associated with intrapulmonary shunting does not respond to supplementary oxygen administration, because the oxygen does not reach the nonventilated alveoli. This finding is in contrast to hypoxemia caused by partially ventilated alveoli (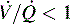 ), which do respond to administration of supplementary oxygen (i.e., the PaO2 increases). An important distinction is that alveoli that are not ventilated (i.e.,
), which do respond to administration of supplementary oxygen (i.e., the PaO2 increases). An important distinction is that alveoli that are not ventilated (i.e., 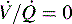 ) must be recruited (opened) for supplementary oxygen to improve oxygenation. With treatment, the
) must be recruited (opened) for supplementary oxygen to improve oxygenation. With treatment, the 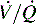 ratio is converted from zero
ratio is converted from zero 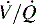 (shunt) to low or normal
(shunt) to low or normal 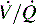 (i.e., to oxygen-responsive hypoxemia).
(i.e., to oxygen-responsive hypoxemia).
During the first days of life, neonates demonstrate both cardiac and intrapulmonary shunting of blood. The cardiovascular shunt is caused by the patent ductus arteriosus with some desaturated pulmonary arterial blood shunted through the ductus into the arterial circulation (the aorta) without passing through the lungs. This right-to-left shunt occurs because pulmonary vascular resistance is high at birth. Once pulmonary vascular resistance begins to fall, the volume of right-to-left shunt falls. In infants with a patent ductus arteriosus, a PaO2 of 60 to 80 mm Hg may be normal in the first day of life, but the PaO2 typically exceeds 80 mm Hg within 2 or 3 days after birth (Table 9-2) as pulmonary vascular resistance falls and the right-to-left shunt ceases. A small amount of right-to-left shunting can also occur in the immediate newborn period through a patent foramen ovale.
Table 9-2 Normal Arterial Blood Gas Values in Children
| Neonate at Birth | Child | |
| pH | 7.32-7.42 | 7.35-7.45 |
| PCO2 | 30-40 mm Hg | 35-45 mm Hg |
| HCO3 | 20-26 mEq/L | 22-28 mEq/L |
| PO2 | 60-80 mm Hg | 80-100 mm Hg |
The neonatal values represent normal values for neonates during the first days of life. Values for the child are the same as for the adult.
Gas Transport
Oxygen Tension and Oxygen Content
Oxygen is carried most efficiently when it is bound to hemoglobin and each gram of hemoglobin is able to carry 1.34 mL oxygen. The total oxygen content is determined by multiplying the hemoglobin (in g/dL) by 1.34 mL O2/g of saturated hemoglobin and then multiplying that number by the actual hemoglobin saturation. The small amount of oxygen carried in the dissolved form is then added to the amount of O2 carried by hemoglobin. The normal arterial O2 content is approximately 18 to 20 mL O2 per dL blood (Box 9-2).271
To emphasize the difference between PaO2 and arterial O2 content, consider the effects of varying hemoglobin concentration in three patients. If the patients all breathe room air, as noted previously, the PaO2 of all patients will equal approximately 110 mm Hg, regardless of hemoglobin concentration. If the three patients have normal lungs, their hemoglobin will be fully saturated (99%), so their total arterial oxygen content will differ according to their hemoglobin concentration. If the first patient has no hemoglobin at all (concentration of 0 g/dL), the patient’s PaO2 is still 110 mm Hg, but the patient’s arterial oxygen content is 0.33 mL/dL (equal to the amount of dissolved oxygen, or 0.003 × PaO2). This example is not realistic, but it makes the point that the PaO2 is not the same as O2 content. Consider a second patient with a hemoglobin concentration of 8 g/dL; the second patient’s PaO2 is 110 mm Hg, with a total arterial oxygen content of approximately 11 mL O2 per dL blood (slightly more than half normal). The third patient has a hemoglobin concentration of 15 g/dL; this patient’s PaO2 is 110 mm Hg, and the patient’s arterial oxygen content is approximately 20 mL O2 per dL blood (normal). Although all three patients have exactly the same PaO2 and oxygen saturation, the second patient must almost double cardiac output to maintain the same oxygen delivery as the third patient (DO2 = CO × CaO2). These examples illustrate the importance of evaluating hemoglobin concentration, PaO2, and arterial oxygen saturation when interpreting blood gas results. Additional patient examples are included in Box 9-3.
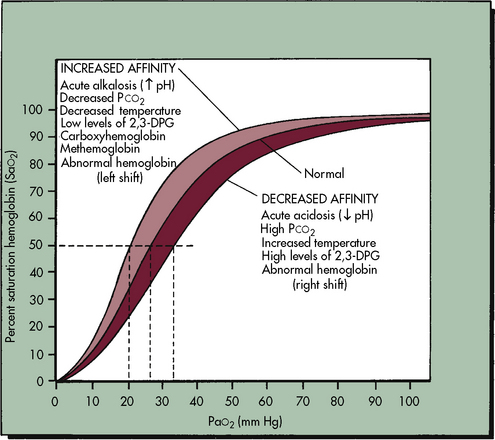
The Oxyhemoglobin Dissociation Curve
The relationship between the PaO2 and the hemoglobin saturation is expressed by the oxyhemoglobin dissociation curve, as shown in Fig. 9-6, with the PaO2 on the horizontal axis and the hemoglobin saturation on the vertical axis. The curve is not linear but S-shaped, with a large plateau at the higher levels of PaO2. There are several important things to note about the oxyhemoglobin dissociation curve. As noted, the curve flattens when the PaO2 exceeds 80 to 100 mm Hg; this means that although the PaO2 continues to rise beyond 100 mm Hg, the hemoglobin cannot become more saturated than 100%, and it cannot carry any more oxygen. Any additional rise in the PaO2 will result only in increases in the amount of dissolved oxygen in the blood, which contributes only 0.003 mL O2 per mm Hg rise in PaO2. Therefore a rise in PaO2 from 100 to 700 torr does not mean that sevenfold more oxygen is carried in the blood; it is associated with an approximately 10% increase in oxygen content. Because the hemoglobin is fully saturated once the PaO2 reaches 100 mm Hg, there is usually no advantage to maintaining the patient’s PaO2 any higher than this value.
As shown in Fig. 9-6, the slope of the oxyhemoglobin dissociation curve becomes extremely steep once the PaO2 is less than 60 mm Hg. Thus when the patient’s PaO2 falls below 60 mm Hg, even small additional decreases in the PaO2 will be associated with a significant fall in the hemoglobin saturation and arterial oxygen content. Therefore the patient’s PaO2 should be maintained above 60 mm Hg, if possible.
The position of the oxyhemoglobin curve can be altered by several factors (Table 9-3). If the curve is shifted to the right, then hemoglobin has less affinity for oxygen (it is less well saturated) at any partial pressure of oxygen (PaO2). Conversely, if the curve is shifted to the left, then hemoglobin has a higher affinity for oxygen (the hemoglobin is better saturated) at any given PaO2.
Table 9-3 Shifts in Hemoglobin Dissociation Curve
| Shift to Left (Higher oxygen affinity) | Shift to Right (Lower oxygen affinity) |
| Alkalosis | Acidosis |
| Hypocapnia | Hypercapnia |
| Hypothermia | Hyperthermia |
| Fetal hemoglobin (decreased 2,3 DPG) | Increased 2,3 DPG |
| Methemoglobinemia | Adult hemoglobin |
Factors that shift the curve to the right include acidosis, hypercapnia, and hyperthermia. Under these conditions the oxyhemoglobin saturation and oxygen content is lower at any given PaO2, but within the normal range the amount of oxygen released to tissues is enhanced, which is an adaptive response that makes oxygen more available in the tissue beds of patients who are likely to need it (e.g., those who are acidotic, hypercapnic, febrile).271 Factors that shift the oxyhemoglobin dissociation curve to the left include alkalosis, hypocapnia, and hypothermia. Although these factors increase oxyhemoglobin saturation at any given PaO2, oxygen will not be as readily released to the tissues,271 because oxygen is more tightly bound to the hemoglobin molecule.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Be sure to check out the supplementary content available at
Be sure to check out the supplementary content available at 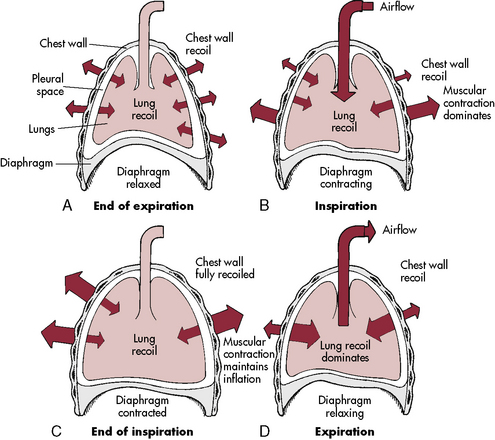
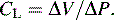
 ) is the product of respiratory frequency and tidal volume:
) is the product of respiratory frequency and tidal volume: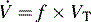
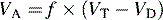
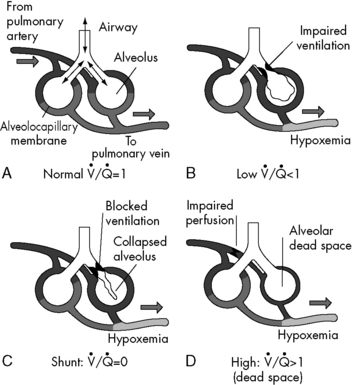
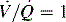 . B and C, Lung diseases characterized by a loss of alveolar volume (e.g., acute respiratory distress syndrome) create V/Q ratios that are either low (
. B and C, Lung diseases characterized by a loss of alveolar volume (e.g., acute respiratory distress syndrome) create V/Q ratios that are either low (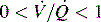 )—shown in B—or zero (
)—shown in B—or zero (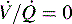 which is the definition for intrapulmonary shunt)—shown in C. Importantly, low
which is the definition for intrapulmonary shunt)—shown in C. Importantly, low 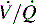 alveolar units are responsive to oxygen administration (i.e., results in an increase in PaO2), whereas
alveolar units are responsive to oxygen administration (i.e., results in an increase in PaO2), whereas 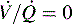 alveolar units (intrapulmonary shunt) are not responsive to oxygen administration. D, High
alveolar units (intrapulmonary shunt) are not responsive to oxygen administration. D, High  units (dead space ventilation) are created under any circumstances in which pulmonary perfusion is reduced while alveolar ventilation is maintained. Thus any clinical condition that decreases right ventricular output (e.g., full cardiac arrest) or increases pulmonary vascular resistance (e.g., excessive PEEP) will result in increased dead space ventilation.
units (dead space ventilation) are created under any circumstances in which pulmonary perfusion is reduced while alveolar ventilation is maintained. Thus any clinical condition that decreases right ventricular output (e.g., full cardiac arrest) or increases pulmonary vascular resistance (e.g., excessive PEEP) will result in increased dead space ventilation.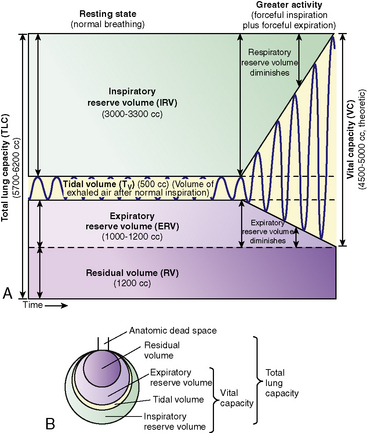
 match are the most common causes of hypoxemia in pediatric lung disorders. These physiologic or abnormal intrapulmonary shunts do not result in hypercapnia (increased PaCO2), because CO2 is highly soluble in capillary blood and rapidly diffuses into the alveoli, and it is eliminated during expiration (exhalation).
match are the most common causes of hypoxemia in pediatric lung disorders. These physiologic or abnormal intrapulmonary shunts do not result in hypercapnia (increased PaCO2), because CO2 is highly soluble in capillary blood and rapidly diffuses into the alveoli, and it is eliminated during expiration (exhalation).