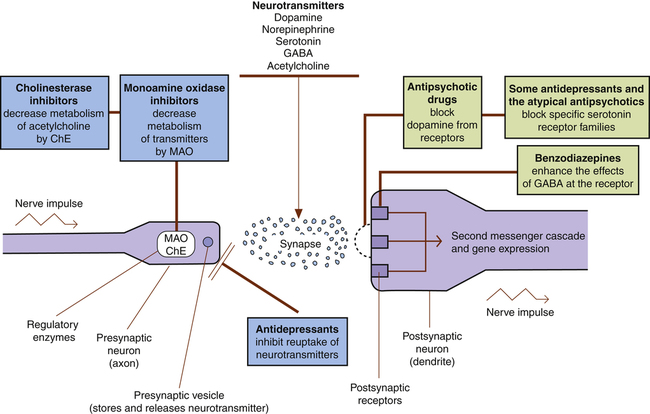
1. Describe the role of the nurse in psychopharmacological treatments. 2. Examine pharmacological actions, drug co-administration, and drug interactions. 3. Analyze the mechanism of action, clinical use, and side effects related to antianxiety, sedative-hypnotic, antidepressant, mood-stabilizing, and antipsychotic drugs. 4. Evaluate the implications of new psychopharmacological agents and genomics. 5. Discuss psychiatric nursing practice issues related to psychopharmacology. This chapter discusses psychopharmacology and important principles of drug therapy in the treatment of patients with neurobiological brain disorders, or mental illnesses. The pharmacological agents described in this chapter are all approved by the U.S. Food and Drug Administration (FDA), although not always for the indications described. Dietary supplements and herbal preparations used to treat the symptoms of mental illness are described in Chapter 30. It is essential that a thorough patient baseline assessment—including history, physical, and laboratory examination (Chapter 5); psychiatric evaluation (Chapter 6); sociocultural assessment (Chapter 7); and a medication history (Box 26-1) be completed for each patient before beginning any treatment. This information helps distinguish aspects of the psychiatric illness from aspects of the patient’s personality that were present before the onset of illness. Finally, careful baseline assessment of each patient can help identify undiagnosed medical illnesses that co-exist with the psychiatric illness or that may be causing the psychiatric symptoms. See Box 26-1 for a medication assessment tool to guide the nurse in taking a drug and substance use history. Some racial and ethnic groups have genetic predispositions toward deficiencies in these enzymes, making them at greater risk for CYP-450 problems. The medication prescriber is responsible for anticipating these possibilities and prescribing accordingly. The nurse should be vigilant for signs of drug effects that seem inconsistent with the doses prescribed or that are adverse reactions (Howland, 2011a). Legislation has been passed in every state in the United States authorizing advanced practice registered nurses (APRNs) to have at least some degree of authority to prescribe medications, and in some states APRNs have full autonomy in this role (Pearson, 2011). Psychiatric–mental health nurse practitioners, and in some states this includes clinical nurse specialists, who are qualified under their state nurse practice acts are thus able to prescribe pharmacological agents to treat the symptoms and improve the functional status of patients with psychiatric illnesses (NONPF, 2011). • Absorption—how the drug is moved into the bloodstream from the site of administration • Distribution—how much drug is moved into various body tissues • Metabolism—how the drug is altered, usually by liver enzymes, into its active and inactive parts • Elimination—how much of the drug is removed from the body in a specific amount of time For example, most of the antidepressants, some of the typical antipsychotics, the mood stabilizers, and even grapefruit juice can inhibit drug-metabolizing liver enzymes in the CYP-450 system, potentially causing toxic levels of other drugs. The mood stabilizer carbamazepine, St. John’s wort, and even smoking cigarettes can markedly reduce many psychotropic drug levels, rendering them ineffective (Fuller and Sajatovic, 2009). Some patients become less responsive to the same dose of a particular drug over time, which is called tolerance, requiring that higher doses of the drug be given over time to obtain the same initial therapeutic effect. The development of tolerance to some drugs, such as BZs or opioids, also may be associated with physical dependence on the drug, requiring tapering during discontinuation to avoid uncomfortable withdrawal symptoms (Chapter 23). • A rebound or recurrence of original symptoms • Uncomfortable new physical and psychological symptoms Gradual tapering from medication can help to prevent this syndrome (Preskorn, 2011). • Box 26-2 lists guidelines for drug co-administration. • Box 26-3 alerts the nurse to patients who may be at higher risk for drug interactions. • Table 26-1 is a reference list for the more common interactions of psychotropic drugs and other substances. TABLE 26-1 INTERACTIONS OF PSYCHOTROPIC DRUGS AND OTHER SUBSTANCES Although this chapter focuses on the adult patient, special populations, such as youth (Chapters 35 and 36), the elderly (Chapter 37), and members of racially and ethnically diverse or disadvantaged groups, are regularly given psychoactive drugs, even though these drugs may not have been adequately tested in randomized clinical trials on these populations. An understanding of relevant issues will help the nurse administer psychopharmacological agents safely to persons who are members of special populations. The FDA categories related to use during pregnancy are as follows: • A—controlled studies show no risk • B—no evidence of risk in humans • D—positive evidence of risk; however, potential benefits may outweigh potential risks The FDA has not approved any psychotropic medication for use during pregnancy or lactation; therefore it is up to the provider and patient to individually weigh the risks and benefits of medication use (Howland, 2009). A careful risk-benefit analysis of the psychiatrically symptomatic mother should include these risks: inattention to prenatal care, poor maternal health, adverse effect on mother-infant bonding, increased stress levels on the fetus and infant, history of adverse drug effects on the fetus, and blood levels of the drug measured in breast milk (Bansil et al, 2010). When the benefits of psychotropic treatment outweigh the risks, some psychotropic drugs may be given during pregnancy and breast-feeding (Meltzer-Brody et al, 2008; Pilowsky et al, 2008). The field of psychopharmacogenetics deals with genetic and environmental factors that control or influence psychotropic drug–metabolizing enzymes, such as the CYP-450 metabolic enzyme system. Differences in the genetically determined structure of these enzymes can account for the ethnic variations that have been reported in drug responses (Chaudhry et al, 2008). The following issues should be considered when prescribing psychotropic drugs for women (Seeman, 2010): • Differences in pharmacokinetics: Gastric emptying is slower, gastric acidity is lower, blood volume is lower, renal clearance of drugs is decreased, and percentage of body fat is higher. Thus women experience greater biological activity than men and often require lower doses of most psychotropic medicines. • Dosage adjustment across the menstrual cycle and after menopause: Pharmacokinetics can differ significantly at different phases of a woman’s menstrual cycle, necessitating dosage adjustment across the cycle for some drugs. Women of reproductive age require lower doses of antipsychotic drugs. Increased prolactin levels caused by some antipsychotic drugs can inhibit ovulation and cause menstrual cycle irregularity. • Interactions between psychotropic agents and prescribed hormones: Oral contraceptives can magnify pharmacokinetic differences, sometimes requiring dosage adjustments. Drugs that induce hepatic enzymes, such as carbamazepine, can increase the metabolism of oral contraceptives, resulting in unwanted pregnancy. In women with bipolar disorder, hormone replacement therapy can trigger rapid cycling. All communication in the brain involves neurotransmission, or neurons “talking” to each other across synapses at receptors. Neurons are the basic functional unit of the brain structures of the nervous system (Chapter 5). The following description is a basic frame of reference from which to understand neuropharmacological mechanisms. The synapse is a narrow gap separating two neurons: the presynaptic cell and the postsynaptic cell (Figure 26-1). Most receptors are three-dimensional “gates” (channels) located on cells (neurons) that are targets for chemical first messengers (e.g., neurotransmitters, peptides, drugs). • Psychosis is thought to involve excessive dopamine and serotonin dysregulation. • Mood disorders are thought to result from disruption of normal patterns of neurotransmission of norepinephrine, serotonin, and other transmitters. • Anxiety is thought to be a dysregulation of GABA and other transmitters. • Alzheimer disease is thought to result from a dysregulation of acetylcholine and other transmitters. • Release: More neurotransmitter is released into the synapse from the storage vesicles in the presynaptic cell. • Blockade of postsynaptic receptors: The neurotransmitter is prevented from binding to the target receptor. • Blockade of alpha2 presynaptic autoreceptors: This negative feedback system is prevented from turning off the release of norepinephrine into the synapse. • Receptor sensitivity changes: The receptor becomes more or less responsive to the neurotransmitter. • Reuptake inhibition: The presynaptic cell does not reabsorb the neurotransmitter well, leaving more neurotransmitter in the synapse and therefore enhancing or prolonging its action. • Interference with storage vesicles: The neurotransmitter is either released again into the synapse (more neurotransmitter) or released to metabolizing enzymes (less neurotransmitter). • Precursor chain interference: The process that makes the neurotransmitter is either facilitated (more is synthesized) or disrupted (less is synthesized). • Synaptic enzyme inhibition: Less neurotransmitter is metabolized, so more remains available in the synapse and the presynaptic neuron. • Second-messenger cascade: A chemical chain reaction within the cell is initiated by neurochemical effects at the receptor during neurotransmission, activating genetically determined brain function. Not all of these strategies have yielded clinically relevant treatments to date. Those that have are emphasized in this chapter (see Figure 26-1): • Antipsychotic drugs block dopamine from the receptor site. • Antidepressants block the reuptake of norepinephrine and/or serotonin and regulate the areas of the brain that manufacture these chemicals. • Monoamine oxidase inhibitors (MAOIs) decrease enzymatic metabolism of norepinephrine and serotonin. • Cholinesterase (ChE) inhibitors decrease the metabolism of acetylcholine. • BZs potentiate (enhance the effects of) GABA. • Some antidepressants and atypical antipsychotics block specific subtypes of serotonin receptors (thought to be responsible for serotonin side effects), thereby enhancing serotonin transmission at serotonin receptors implicated in depression. Anxiety is a normal response to threat and is part of the fight-or-flight instinct necessary for survival (Chapter 15). The diagnosis of anxiety (symptoms of anxiety that are disproportionate to the circumstances) is based on the patient’s description, the nurse’s observation of behaviors, assessment of DSM-IV-TR (APA, 2000) diagnostic criteria, and the elimination of alternative diagnoses. Used in higher doses, the high-potency BZs alprazolam and clonazepam have been effective in the treatment of panic disorder and social phobia. The target symptoms for the use of BZs are listed in Box 26-4. Another clinical indication for the use of BZs is as a sedative-hypnotic to improve sleep. Insomnia includes difficulty falling asleep, difficulty staying asleep, or awakening too early with an inability to go back to sleep. It is a symptom with many causes and often responds to nonpharmacological strategies, such as talking about problems, increased daytime exercise, elimination of stimulants such as caffeine, and incorporating physical comfort measures into the nighttime routine (Chapter 16). Because the BZs are in the same pharmacological class as alcohol, they can be used to suppress the alcohol withdrawal syndrome and are the treatment of choice for this indication (Chapter 23). The ingestion of these two substances together is contraindicated, particularly for the patient using dangerous equipment or driving a car, because it can produce extreme sedation. The BZs have no significant clinical advantages over each other, although differences in half-life can be clinically useful (Table 26-2). For example, patients with persistent high levels of anxiety should take a drug with a long half-life. Patients with fluctuating anxiety might do better with either a short-acting drug or a drug with a sustained-release formulation (alprazolam, clorazepate, diazepam). Sustained-release BZs blunt the peaks of toxicity and the lows of symptom breakthrough and are a popular alternative to the original formulations. TABLE 26-2 ANTIANXIETY AND SEDATIVE-HYPNOTIC DRUGS: BENZODIAZEPINES ∗Dosage ranges are approximate and should be individualized for each patient. BZ side effects are common, dose related, usually short-term, and almost always harmless. Table 26-3 summarizes these reactions and nursing considerations. TABLE 26-3 BENZODIAZEPINE SIDE EFFECTS AND NURSING CONSIDERATIONS Tolerance can develop to the sedative effects of BZs, but it is unclear whether tolerance also develops to induced sleep or antianxiety effects. These drugs should be tapered to minimize withdrawal symptoms (Box 26-5) and rebound symptoms of insomnia or anxiety. If these symptoms occur, the dose should be raised until symptoms are gone and then tapering resumed at a slower rate. Elderly patients are more vulnerable to side effects because the aging brain is more sensitive to sedatives. Dosing ranges from one half to one third of the usual daily dose used for adults. The BZs with no active metabolites (see Table 26-2) are less affected by liver disease, the age of the patient, or drug interactions. Buspirone, a non-BZ anxiolytic drug, is a potent antianxiety agent with no addictive potential and has FDA approval for the treatment of GAD (Table 26-4). Buspirone does not exhibit muscle-relaxant or anticonvulsant activity, interaction with CNS depressants, or sedative-hypnotic properties. It is not effective in the management of drug or alcohol withdrawal or panic disorder. Generally it takes several weeks for buspirone’s antianxiety effects to take effect. It probably is most effective in patients who have never taken BZs and therefore are not expecting immediate effects from drug treatment. TABLE 26-4 NONBENZODIAZEPINE ANTIANXIETY AND SEDATIVE-HYPNOTIC AGENTS Clonidine is also used to block physiological symptoms of opioid withdrawal and the tachycardia and excessive salivation seen with the atypical antipsychotic clozapine (Schatzberg et al, 2010). • Duloxetine: generalized anxiety disorder (GAD) • Fluoxetine: obsessive-compulsive disorder (OCD), panic disorder, bulimia, and premenstrual dysphoric disorder (PMDD) • Paroxetine: OCD, panic disorder, PMDD, social anxiety disorder (SAD), GAD, and posttraumatic stress disorder (PTSD) • Sertraline: OCD, panic disorder, PMDD, SAD, and PTSD
Psychopharmacology
Role of the Nurse in Psychopharmacology
Patient Assessment
Monitoring Drug Effects
Prescriptive Authority
Pharmacological Principles
Pharmacokinetics
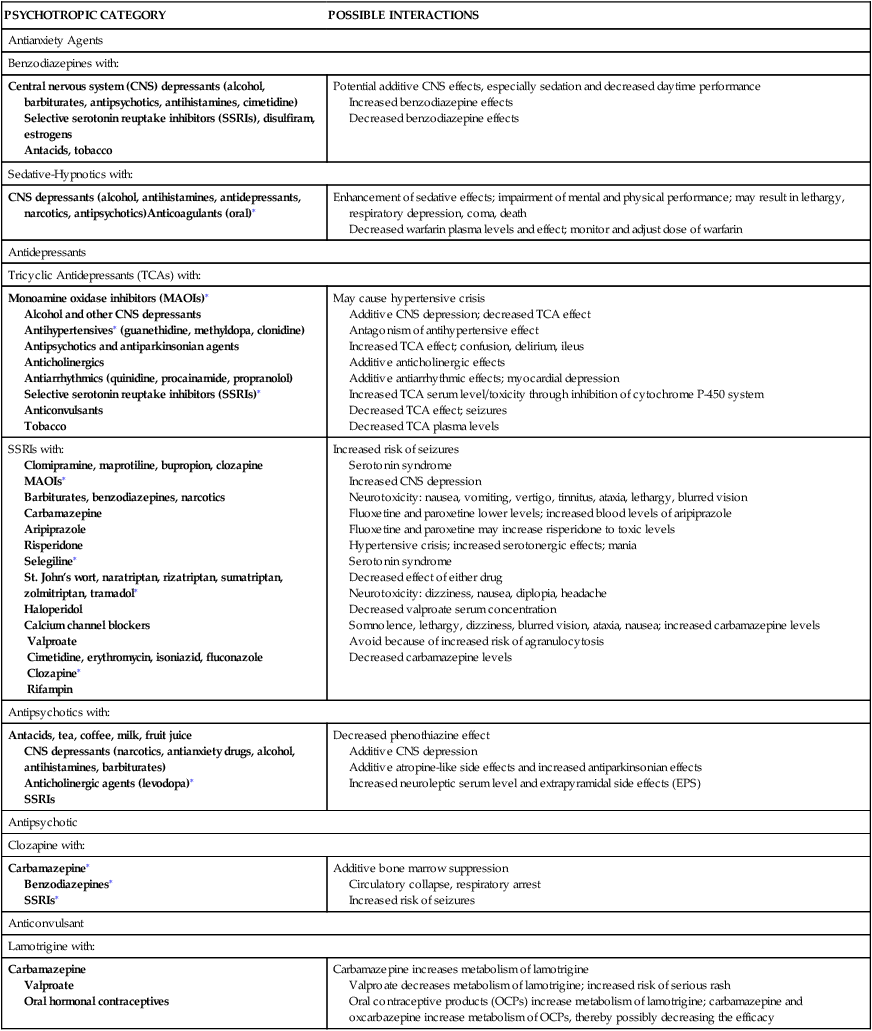
Drug Interactions
Pharmacodynamics
Tolerance, Dependence, and Withdrawal Symptoms
Drug Co-Administration
PSYCHOTROPIC CATEGORY
POSSIBLE INTERACTIONS
Antianxiety Agents
Benzodiazepines with:
Central nervous system (CNS) depressants (alcohol, barbiturates, antipsychotics, antihistamines, cimetidine)
Selective serotonin reuptake inhibitors (SSRIs), disulfiram, estrogens
Antacids, tobacco
Potential additive CNS effects, especially sedation and decreased daytime performance
Increased benzodiazepine effects
Decreased benzodiazepine effects
Sedative-Hypnotics with:
CNS depressants (alcohol, antihistamines, antidepressants, narcotics, antipsychotics)Anticoagulants (oral)∗
Enhancement of sedative effects; impairment of mental and physical performance; may result in lethargy, respiratory depression, coma, death
Decreased warfarin plasma levels and effect; monitor and adjust dose of warfarin
Antidepressants
Tricyclic Antidepressants (TCAs) with:
Monoamine oxidase inhibitors (MAOIs)∗
Alcohol and other CNS depressants
Antihypertensives∗ (guanethidine, methyldopa, clonidine)
Antipsychotics and antiparkinsonian agents
Anticholinergics
Antiarrhythmics (quinidine, procainamide, propranolol)
Selective serotonin reuptake inhibitors (SSRIs)∗
Anticonvulsants
Tobacco
May cause hypertensive crisis
Additive CNS depression; decreased TCA effect
Antagonism of antihypertensive effect
Increased TCA effect; confusion, delirium, ileus
Additive anticholinergic effects
Additive antiarrhythmic effects; myocardial depression
Increased TCA serum level/toxicity through inhibition of cytochrome P-450 system
Decreased TCA effect; seizures
Decreased TCA plasma levels
SSRIs with:
Clomipramine, maprotiline, bupropion, clozapine
MAOIs∗
Barbiturates, benzodiazepines, narcotics
Carbamazepine
Aripiprazole
Risperidone
Selegiline∗
St. John’s wort, naratriptan, rizatriptan, sumatriptan, zolmitriptan, tramadol∗
Haloperidol
Calcium channel blockers
Valproate
Cimetidine, erythromycin, isoniazid, fluconazole
Clozapine∗
Rifampin
Increased risk of seizures
Serotonin syndrome
Increased CNS depression
Neurotoxicity: nausea, vomiting, vertigo, tinnitus, ataxia, lethargy, blurred vision
Fluoxetine and paroxetine lower levels; increased blood levels of aripiprazole
Fluoxetine and paroxetine may increase risperidone to toxic levels
Hypertensive crisis; increased serotonergic effects; mania
Serotonin syndrome
Decreased effect of either drug
Neurotoxicity: dizziness, nausea, diplopia, headache
Decreased valproate serum concentration
Somnolence, lethargy, dizziness, blurred vision, ataxia, nausea; increased carbamazepine levels
Avoid because of increased risk of agranulocytosis
Decreased carbamazepine levels
Antipsychotics with:
Antacids, tea, coffee, milk, fruit juice
CNS depressants (narcotics, antianxiety drugs, alcohol, antihistamines, barbiturates)
Anticholinergic agents (levodopa)∗
SSRIs
Decreased phenothiazine effect
Additive CNS depression
Additive atropine-like side effects and increased antiparkinsonian effects
Increased neuroleptic serum level and extrapyramidal side effects (EPS)
Antipsychotic
Clozapine with:
Carbamazepine∗
Benzodiazepines∗
SSRIs∗
Additive bone marrow suppression
Circulatory collapse, respiratory arrest
Increased risk of seizures
Anticonvulsant
Lamotrigine with:
Carbamazepine
Valproate
Oral hormonal contraceptives
Carbamazepine increases metabolism of lamotrigine
Valproate decreases metabolism of lamotrigine; increased risk of serious rash
Oral contraceptive products (OCPs) increase metabolism of lamotrigine; carbamazepine and oxcarbazepine increase metabolism of OCPs, thereby possibly decreasing the efficacy
Special Populations
Pregnant and Lactating Women
Cross-Cultural Perspectives, Ethnopsychopharmacology, and Gender
Biological Basis for Psychopharmacology
Antianxiety and Sedative-Hypnotic Drugs
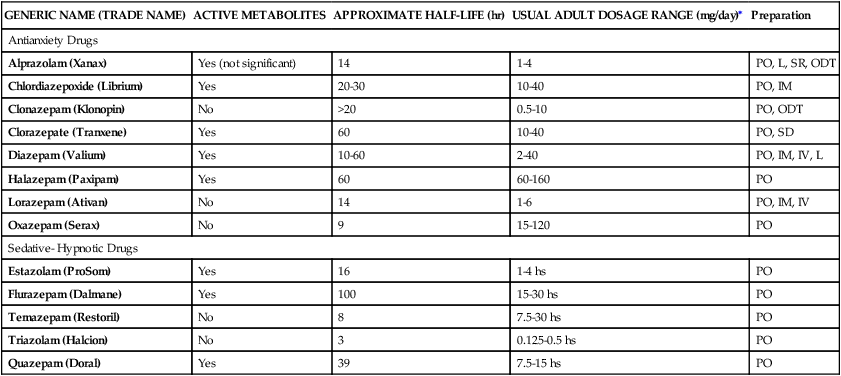
Benzodiazepines
Clinical Use
GENERIC NAME (TRADE NAME)
ACTIVE METABOLITES
APPROXIMATE HALF-LIFE (hr)
USUAL ADULT DOSAGE RANGE (mg/day)∗
Preparation
Antianxiety Drugs
Alprazolam (Xanax)
Yes (not significant)
14
1-4
PO, L, SR, ODT
Chlordiazepoxide (Librium)
Yes
20-30
10-40
PO, IM
Clonazepam (Klonopin)
No
>20
0.5-10
PO, ODT
Clorazepate (Tranxene)
Yes
60
10-40
PO, SD
Diazepam (Valium)
Yes
10-60
2-40
PO, IM, IV, L
Halazepam (Paxipam)
Yes
60
60-160
PO
Lorazepam (Ativan)
No
14
1-6
PO, IM, IV
Oxazepam (Serax)
No
9
15-120
PO
Sedative- Hypnotic Drugs
Estazolam (ProSom)
Yes
16
1-4 hs
PO
Flurazepam (Dalmane)
Yes
100
15-30 hs
PO
Temazepam (Restoril)
No
8
7.5-30 hs
PO
Triazolam (Halcion)
No
3
0.125-0.5 hs
PO
Quazepam (Doral)
Yes
39
7.5-15 hs
PO
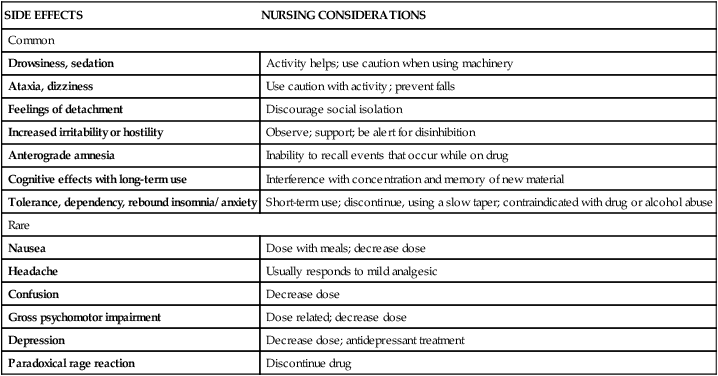
Side Effects and Adverse Reactions
SIDE EFFECTS
NURSING CONSIDERATIONS
Common
Drowsiness, sedation
Activity helps; use caution when using machinery
Ataxia, dizziness
Use caution with activity; prevent falls
Feelings of detachment
Discourage social isolation
Increased irritability or hostility
Observe; support; be alert for disinhibition
Anterograde amnesia
Inability to recall events that occur while on drug
Cognitive effects with long-term use
Interference with concentration and memory of new material
Tolerance, dependency, rebound insomnia/ anxiety
Short-term use; discontinue, using a slow taper; contraindicated with drug or alcohol abuse
Rare
Nausea
Dose with meals; decrease dose
Headache
Usually responds to mild analgesic
Confusion
Decrease dose
Gross psychomotor impairment
Dose related; decrease dose
Depression
Decrease dose; antidepressant treatment
Paradoxical rage reaction
Discontinue drug
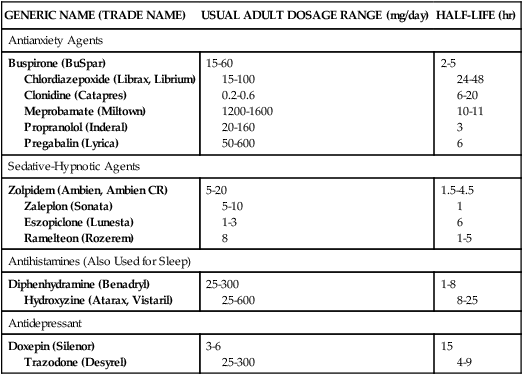
Nonbenzodiazepine Antianxiety Agents
GENERIC NAME (TRADE NAME)
USUAL ADULT DOSAGE RANGE (mg/day)
HALF-LIFE (hr)
Antianxiety Agents
Buspirone (BuSpar)
Chlordiazepoxide (Librax, Librium)
Clonidine (Catapres)
Meprobamate (Miltown)
Propranolol (Inderal)
Pregabalin (Lyrica)
15-60
15-100
0.2-0.6
1200-1600
20-160
50-600
2-5
24-48
6-20
10-11
3
6
Sedative-Hypnotic Agents
Zolpidem (Ambien, Ambien CR)
Zaleplon (Sonata)
Eszopiclone (Lunesta)
Ramelteon (Rozerem)
5-20
5-10
1-3
8
1.5-4.5
1
6
1-5
Antihistamines (Also Used for Sleep)
Diphenhydramine (Benadryl)
Hydroxyzine (Atarax, Vistaril)
25-300
25-600
1-8
8-25
Antidepressant
Doxepin (Silenor)
Trazodone (Desyrel)
3-6
25-300
15
4-9
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Psychopharmacology
Get Clinical Tree app for offline access