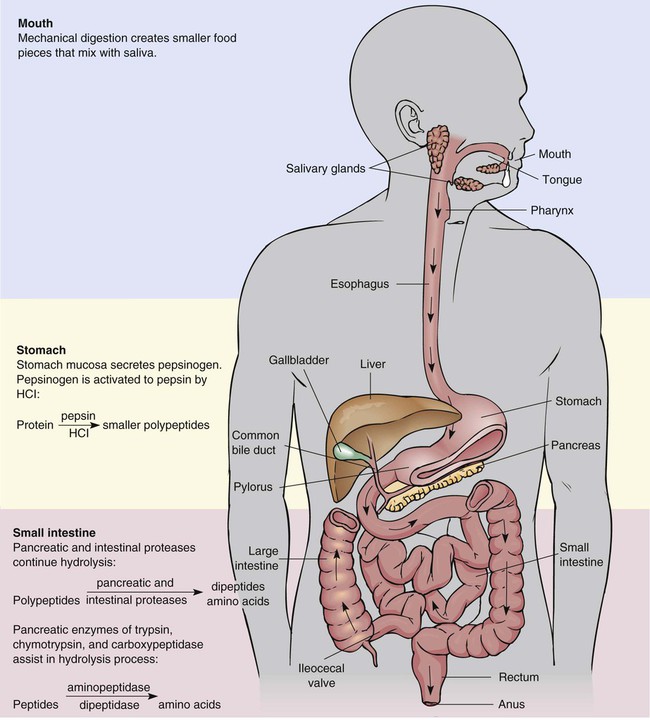
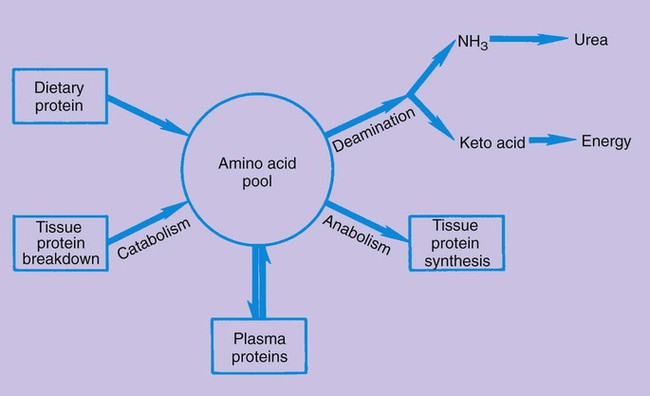
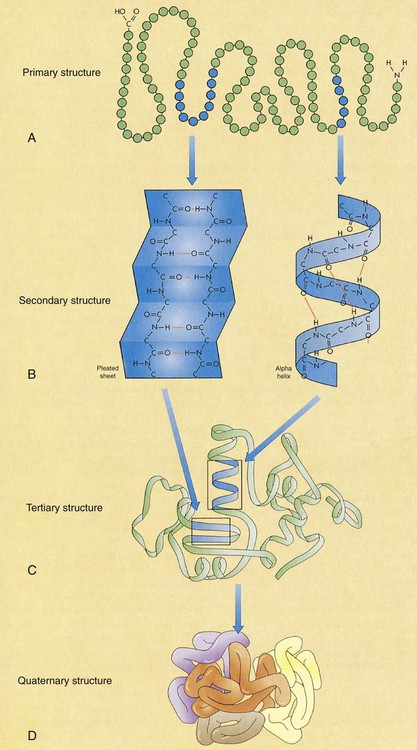
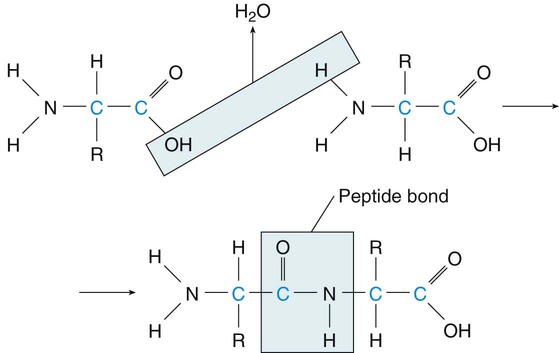
Although proteins formed by our bodies do have a role in those functions, the amounts we consume are often greater than we need. Awareness of protein sources and portion sizes is important as we work toward achieving health promotion goals to decrease our risk of diet-related diseases (Box 6-1). There are 20 amino acids from which all the proteins that are required by plants and animals are made. The human body is able to manufacture some of the amino acids for its own protein-building function; however, 9 amino acids cannot be made by the cells of the body. Therefore, these essential amino acids (EAAs) must be eaten in food, digested, absorbed, and then brought to cells by circulating blood. The remaining 11 are non-essential amino acids (NEAAs) (Box 6-2). The liver can create NEAAs as long as structural components, including nitrogen, from other amino acids are available. The functions of proteins are closely related to their structures. The complex composition of proteins is best understood through four structural levels: primary, secondary, tertiary, and quaternary1 (Figure 6-1). The primary structure of protein composition is determined by the number, assortment, and sequence of amino acids in polypeptide chains. Amino acids are linked together by peptide bonds to form a practically unlimited number of proteins. The peptide bond occurs at the point at which the carboxyl group of one amino acid is bound to the amino group of another amino acid (Figure 6-2). Because of the complex structure of proteins, a number of protein enzymes, or proteases, produced by the stomach and pancreas are required to hydrolyze proteins into smaller and smaller peptides until individual amino acids are ready for absorption (Figure 6-3). To understand the importance of protein metabolism in the growth and maintenance of the body, consider that most protein functions are a result of protein anabolism (synthesis) in cells. Hormones have a major role in the regulation of protein metabolism. Anabolism is enhanced by the effect of growth hormone (from the pituitary gland) and the male hormone testosterone. Hormones affecting the catabolism (break down) of proteins are the glucocorticoids that are enhanced by adrenocorticotropic hormone (ACTH); these hormones are secreted from the adrenal cortex. This process releases proteins in the cells to break down to amino acids, and then the amino acids travel in the bloodstream, contributing to an available pool of amino acids (Figure 6-4). The liver cells begin the process of catabolism through deamination. Deamination results in an amino acid (NH2) group breaking off from an amino acid molecule, resulting in one molecule each of ammonia (NH3) and a keto acid. Liver cells convert most of the ammonia to urea, which is later excreted in urine. The keto acid may enter the tricarboxylic acid (TCA) cycle to be used for energy (see Figure 9-2) or, through gluconeogenesis and lipogenesis, be converted to glucose and fat1 (see Figure 6-4). In fact, the source of excess protein may be a health concern. Animal-derived protein sources such as meats may also be high in saturated fat and cholesterol. This may increase the risk of coronary artery disease (CAD) and some cancers. The relationship between protein intake and osteoporosis also has been considered. When protein intake is high, there is a slight increase of calcium excretion from the body, but calcium absorption is not affected. Studies have yielded mixed results about this effect on the risk of osteoporosis. Because osteoporosis is multifactorial, this specific relationship is difficult to determine. Recommendations to consume moderate amounts of protein and to meet the new Dietary Reference Intake (DRI) levels for calcium are the best dietary approaches to decrease the risk of CAD and cancer. (See Chapter 8 for an in-depth discussion of osteoporosis.) Nitrogen-balance studies are used to determine the protein requirements of the body throughout the life cycle and to assign value to the protein quality of foods to determine their biologic value.2 Because nitrogen (N) is a primary component of protein, the body’s use of protein can be determined by nitrogen-balance studies that compare the amount of nitrogen entering the body in food protein with the nitrogen lost from the body in feces and urine. Proteins created in our bodies perform numerous functions, including the following: Incomplete protein lacks one or more of the nine essential amino acids. These proteins will not provide a sufficient supply of amino acids and will not support life (Box 6-4). Many plant foods contain considerable amounts of incomplete proteins. Some of the better sources are grains and legumes.
Protein
Role in Wellness
![]() http://evolve.elsevier.com/Grodner/foundations/
http://evolve.elsevier.com/Grodner/foundations/ ![]() Nutrition Concepts Online
Nutrition Concepts Online
Structure of Protein
Protein Composition
Protein as a Nutrient in the Body
Digestion and Absorption
Metabolism
Protein Excess
Nitrogen Balance
Functions
Food Sources
Quality of Protein Foods
Protein
Get Clinical Tree app for offline access

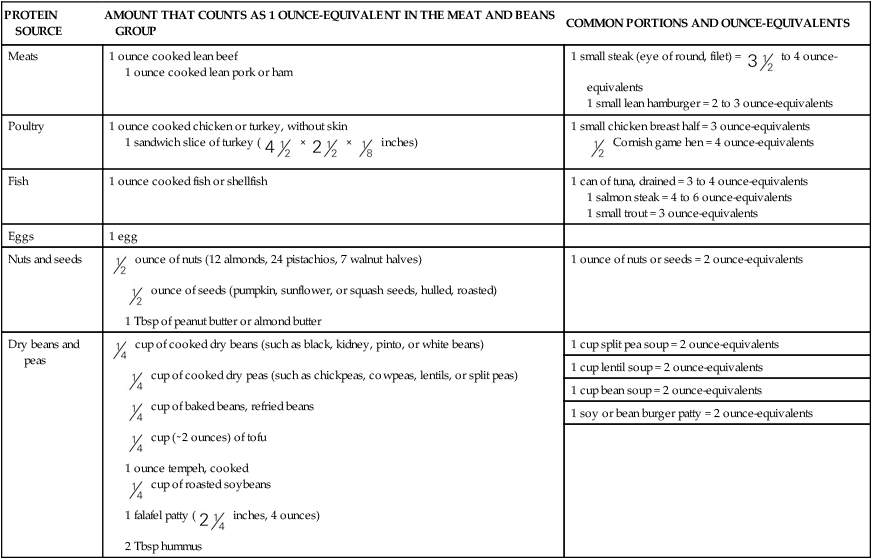
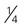 cup of cooked dry beans; 1 egg; 1 tbsp of peanut butter; or
cup of cooked dry beans; 1 egg; 1 tbsp of peanut butter; or 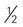 ounce of nuts or seeds can be considered as 1 ounce-equivalent from the meat and beans group.
ounce of nuts or seeds can be considered as 1 ounce-equivalent from the meat and beans group.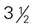 to 4 ounce-equivalents
to 4 ounce-equivalents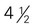 ×
× 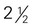 ×
× 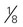 inches)
inches)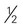 Cornish game hen = 4 ounce-equivalents
Cornish game hen = 4 ounce-equivalents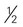 ounce of nuts (12 almonds, 24 pistachios, 7 walnut halves)
ounce of nuts (12 almonds, 24 pistachios, 7 walnut halves)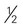 ounce of seeds (pumpkin, sunflower, or squash seeds, hulled, roasted)
ounce of seeds (pumpkin, sunflower, or squash seeds, hulled, roasted)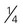 cup of cooked dry beans (such as black, kidney, pinto, or white beans)
cup of cooked dry beans (such as black, kidney, pinto, or white beans)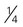 cup of cooked dry peas (such as chickpeas, cowpeas, lentils, or split peas)
cup of cooked dry peas (such as chickpeas, cowpeas, lentils, or split peas)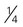 cup of baked beans, refried beans
cup of baked beans, refried beans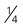 cup (∼2 ounces) of tofu
cup (∼2 ounces) of tofu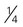 cup of roasted soybeans
cup of roasted soybeans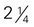 inches, 4 ounces)
inches, 4 ounces)