leukotriene C4 > leukotriene E4. Cys-leukotriene 1 receptors are expressed in airway smooth muscle, tissue macrophages, monocytes, and eosinophils, and cys-leukotriene 2 receptors are found in airway macrophages and smooth muscle cells (Lynch et al., 1999; Spahr & Krawiec, 2008).
Leukotriene receptor antagonists (montelukast and zafirlukast) are LTD4 inhibitors at the CysLT1 receptor and exert their beneficial effects in asthma by antagonizing the detrimental effects of the cysteinyl leukotrienes in airways. Leukotriene sysnthesis inhibitors, also called 5-lipoxygenase inhibitors (zileuton), block the formation of leukotrienes at the earliest step by inhibiting the action of the 5-lipoxygenase enzyme. Beneficial effects of leukotriene modifiers (CysLT1 receptor inhibitors and synthsis inhibitors) include improved lung function, reduced β2-agonist use, attenuated exercise-induced bronchospasm, improved asthma symptoms, reduced use of inhaled corticosteroids, improved quality of life, reduced circulating levels of blood eosinophils, and reduced levels of exhaled nitric oxide (Blake, 1999; Currie & McLaughlin, 2006; Kelly, 2007).
Common Indications
Montelukast (an LTD4 inhibitor at the CysLT1 receptor) is indicated for the prophylaxis and chronic treatment of asthma in patients 12 months of age and older and for the acute prevention of exercise-induced bronchoconstriction in patients 15 years of age and older. It is also indicated for the relief of symptoms of allergic rhinitis (seasonal allergic rhinitis in patients 2 years of age and older, and perennial allergic rhinitis in patients 6 months of age and older). Patients with asthma and allergic rhinitis may receive benefit for both conditions when using montelukast. Zafirlukast (an LTD4 inhibitor at the CysLT1 receptor) is indicated for the prophylaxis and chronic treatment of asthma in adults and children 5 years of age and older. Zileuton (a 5-lipoxygenase inhibitor) is indicated for the prophylaxis and chronic treatment of asthma in adults and in children 12 years of age and older.
The Guidelines for the Diagnosis and Management of Asthma state that leukotriene receptor antagonists are indicated as an alternative to low-dose inhaled corticosteroids for patients with mild persistent asthma and are recommended as add-on treatment to inhaled corticosteroids after consideration of either increasing the inhaled corticosteroid dose or adding a long-acting β2-agonist to inhaled corticosteroid in patients with moderate to severe persistent asthma (EPR 3, 2007). Despite differences in the mechanism of action, there is no evidence for clinical differences between 5-lipoxygenase inhibitors and leukotriene receptor antagonists (Kelly, 2007).
Common Adverse Effects
Common but not serious adverse effects include headache, abdominal pain, pharyngitis, influenza, fever, sinusitis, nausea, diarrhea, dyspepsia, otitis media, viral infection, and laryngitis.
All the leukotriene modifiers have been associated with the occurrence of Churg–Strauss syndrome. Occurrence of Churg–Strauss syndrome is characterized by eosinophilic vasculitis and possible cardiopulmonary complications (Harrold et al., 2007; Lilly et al., 2002). This syndrome appears to manifest in individuals with asthma who were previously controlled with oral corticosteroids and who were weaned from their use after the introduction of a leukotriene modifier or inhaled corticosteroid therapy (Lilly et al., 2002). No causal association between leukotriene modifiers or inhaled corticosteroid therapy and the development of Churg–Strauss syndrome has been established. This event is rare but would be considered serious.
Recently, reports of behavior changes related to leukotriene modifier use has created concern and prompted warnings to be included in the prescribing information. The labeling for leukotriene modifiers now contains language stating “agitation, aggressive behavior or hostility, anxiousness, depression, dream abnormalities, hallucinations, insomnia, irritability, restlessness, somnambulism, suicidal thinking and behavior (including suicide), and tremor may occur.” Analyses from two recent publications involving over 20,000 patients treated with montelukast found no evidence of “possibly suicidality related adverse events” nor “behavior-related adverse events” (Philip et al., 2009a, 2009b). In addition, analysis of three recent large asthma trials conducted by the American Lung Association Asthma Clinical Research Centers network in 569 patients treated with montelukast has uncovered no behavioral problems (Holbrook & Harik-Khan, 2008). These events are rare but would be considered serious.
Zileuton can cause liver dysfunction, and liver function monitoring is currently recommended before treatment begins: once a month for the first 3 months, every two to three months for the remainder of the first year, and periodically thereafter for patients receiving long-term therapy (Zileuton Prescribing Information, 2009).
Zafirlukast can also cause liver dysfunction. Periodic serum transaminase testing has not been proven to prevent serious injury, but it is generally believed that early detection of drug-induced hepatic injury along with immediate withdrawal of the suspect drug enhances the likelihood for recovery (Zafirlukast Prescribing Information, 2009).
Generic and Brand Names
Leukotriene modifiers are available as montelukast (Singulair), zafirlukast (Accolate), and zileuton (Zyflo and Zyflo CR).
Formulations
All leukotriene modifiers are available as tablets for oral ingestion. Montelukast (Singulair) is also available as a chewable tablet and as granules (to be sprinkled on food for young children).
OTHER ANTI-INFLAMMATORY DRUGS AND IMMUNE MODULATORS
Cromolyn
Mechanism of Action
The exact mechanisms of action for cromolyn is largely unknown, but it inhibits IgE mediated release of mediators from mast cells and also inhibits the release of mediators from eosinophils, alveolar macrophages, neutrophils, and monocytes (Brogden & Sorkin, 1993). This effect varies depending on the species and the cell type tested (Kelly, 1999a). Cromolyn prevents mast cell degranulation induced by nonimmunologic stimuli, such as phospholipase A, dextran, and polymyxin B. This effect likely involves regulation of intracellular calcium probably by phosphorylation of a specific membrane protein, which inhibits calcium influx into the cell. Other nonspecific effects include inhibition of phosphodiesterase, modification of the vagal reflex, inhibition of irritant receptors, chemotaxis inhibition of inflammatory mediators, and possibly inhibition of inflammatory neuropeptide release, which induce bronchoconstriction through efferent cholinergic pathways (Kelly, 1999a).
Common Indications
Cromolyn is indicated for the prophylaxis of asthma symptoms. The solution for nebulization is indicated for patients 2 years and older. It is not indicated for the relief of symptoms from acute bronchoconstriction.
Common Adverse Effects
Adverse effects include transient bronchospasm, cough, bad taste, and nausea. Bronchospasm is quickly relieved with the administration of an inhaled β2-agonist.
Generic and Brand Names
The only available cromolyn products are solution for nebulization (various generics).
Formulations
Cromolyn is available as a solution for nebulization.
Omalizumab
Mechanism of Action
Omalizumab is an anti-IgE monoclonal antibody. IgE plays a critical role in the inflammatory process of allergic asthma, and high-affinity receptors for circulating IgE (Fcε-R1) are found on mast cells and basophils. Allergens inhaled into the lung cross-link IgE bound to mast cells and basophils, which causes the mast cells and basophils to degranulate and release preformed mediators (histamine and tryptase) and rapidly synthesized mediators (bradykinin, prostaglandin E2, prostaglandin F2, and leukotrienes). Omalizumab binds to the Cε3 domain of free IgE in the serum and not to IgE already bound to mast cells. The omalizumab–IgE complex prevents IgE from binding to the Fcε-R1 on mast cells and basophils. Omalizumab also downregulates Fcε-R1 receptor expression on basophils, which further dampens the allergic response (Holgate et al., 2005). Serum IgE levels are not to be measured for monitoring purposes, as most clinical assays do not distinguish between free and bound IgE in the serum (Ruffin & Busch, 2004).
Common Indications
Omalizumab is indicated for patients with moderate to severe persistent asthma who have had a positive skin test or in vitro reactivity to a perennial aeroallergen and symptoms that are inadequately controlled with inhaled corticosteroids (Omalizumab Prescribing Information, 2010). It is not indicated for other allergic conditions, acute bronchospasm, or for patients less than 12 years of age.
Common Adverse Effects
The most common adverse reactions are arthralgia and pain. Injection site reactions including bruising, redness, warmth, burning, stinging, itching, hive formation, pain, indurations, mass, and inflammation can occur within 1 hour of injection and may last up to 8 days. Injection site reactions tend to decrease with continued treatment (Omalizumab Prescribing Information, 2010).
Anaphylaxis is a potential adverse effect; thus, patients should be observed for a period of time after dosing. The frequency of anaphylaxis attributed to omalizumab is estimated to be at least 0.2% of patients (Omalizumab Prescribing Information, 2010). Signs and symptoms in these reported cases have included bronchospasm, hypotension, syncope, urticaria, and/or angioedema of the throat or tongue (Omalizumab Prescribing Information, 2010). Anaphylaxis has occurred as early as after the first dose of omalizumab but also has occurred beyond 1 year after beginning regularly scheduled treatment (Omalizumab Prescribing Information, 2010). Symptoms may begin within 2 hours after the dose.
Malignant neoplasms have been reported with omalizumab use and were observed in 20 of 4,127 (0.5%) Xolair-treated patients compared with 5 of 2,236 (0.2%) control patients in clinical studies of adults and adolescents with asthma and other allergic disorders (Omalizumab Prescribing Information, 2010). The observed malignancies in omalizumab-treated patients included breast, nonmelanoma skin, prostate, melanoma, and parotid, which occurred more than once, and five other types, which occurred only once each (Omalizumab Prescribing Information, 2010). An independent panel concluded that these findings did not suggest a neoplastic risk with omalizumab, though most patients were observed for only about 1 year (Corren et al., 2009). However, a 5-year study is in progress to monitor for these effects.
There is a possible increased risk for parasitic (helminth) infections with omalizumab treatment. Patients at high risk of geohelminth infection should be monitored during and after stopping omalizumab treatment (Omalizumab Prescribing Information, 2010).
Generic and Brand Names
The only IgE monoclonal antibody available is omalizumab (Xolair).
Formulations
Omalizumab is available as a lyophilized, sterile powder for subcutaneous injection.
COUGH SUPPRESSANTS
Dextromethorphan
Mechanism of Action
Dextromethorphan is the methyl ether d-isomer of the codeine analogue levorphanol but lacks typical opiate agonist characteristics aside from its antitussive activity. The exact mechanism of action is in dispute but may have several mechanisms for cough suppressant effect, including glutamate receptor antagonism (specifically, N-methyl-D-aspartate receptor), sigma-1 receptor agonism, serotonin pathway agonism, and effects on the cough gating mechanism (Bolser, 2006; Canning, 2009; Bolser, 2009; Eccles, 2009).
Common Indications
Dextromethorphan is indicated for the temporary relief of nonproductive cough.
Common Adverse Effects
Common adverse effects are mild and infrequent and can include dizziness, fatigue, gastrointestinal disturbances, and drowsiness. Dextromethorphan has been associated with serotonergic effects and at higher doses may cause confusion, nervousness, restlessness, dysarthria, irritability, nausea and vomiting.
Some dextromethorphan-containing products contain tartrazine, which may cause allergic-type reactions (including asthma symptoms) in patients with a specific sensitivity (Ardern & Ram, 2001). Although the overall prevalence of tartrazine sensitivity in the general population is low, it may occur in patients who also have aspirin hypersensitivity, though this was disputed in a recent study evaluating tartrazine sensitivity in patients with nonsteroid anti-inflammatory drug sensitivity (Pestana, Moreira, & Olej, 2010).
Beginning in 2007, the Centers for Disease Control issued warnings for the risk of serious injury and fatal overdose from cough and cold products administered to children less than 2 years old. Subsequently, the Food and Drug Administration Nonprescription Drug Advisory Committee and Pediatric Advisory Committee recommended that products containing dextromethorphan, pseudoephedrine, chlorpheniramine, diphenydramine, brompheniramine, phenylephrine, clemastine, and guaifenesin not be used in children less than 6 years old. The Food and Drug Administration issued a Public Health Advisory recommending that nonprescription products (though prescription products also containing these ingredients would be included) not be used in children under 2 years old. Further official rulings from the Food and Drug Administration are expected.
Generic and Brand Names
There are numerous brand and generic products available without prescription.
Formulations
Dextromethorphan is available in liquid, tablet, softgel, and dissolving film formulations.
Codeine
Mechanism of Action
Codeine is a narcotic opioid that acts centrally in the cough center in the medulla by binding primarily to μ-opioid receptors and possibly κ-opioid receptors (Takahama & Shirasaki, 2007). Codeine also has a drying effect on the mucosa of the respiratory tract and increases the viscosity of bronchial secretions.
Common Indications
Codeine is indicated for the temporary relief of nonproductive cough.
Common Adverse Effects
The most serious adverse effects include respiratory depression and arrest, circulatory depression, shock, and cardiac arrest. Codeine can also cause drowsiness, dizziness, confusion, insomnia, nervousness, dysphoria, euphoria, mood alterations, and anxiety. Gastrointestinal effects may include anorexia, nausea, vomiting, and constipation.
Due to histamine release, codeine can cause flushing and pruritus. Anticholinergic effects are infrequent but can include sinus bradycardia, sinus tachycardia, changes in blood pressure, and syncope, as well as dry mouth, blurred vision, or urinary retention. Miosis can occur at therapeutic doses.
Also see the information regarding use in children from the Centers for Disease Control and Food and Drug Administration previously discussed under the section “Dextromethorphan.”
Generic and Brand Names
Codeine-containing cough suppressant products are Schedule V. Numerous brand and generic products are available and distributed at retail without prescription, but only by a registered pharmacist; in some states, a prescription may be required.
Formulations
Products are available as tablets and oral liquids alone or in combination with expectorants and decongestants.
Diphenydramine, Promethazine, and Other First-Generation Antihistamines
Mechanism of Action
First-generation antihistamines such as diphenhydramine and promethazine have direct suppressive actions on the cough center in the medulla.
Common Indications
First-generation antihistamines can be used for the temporary relief of nonproductive cough.
Common Adverse Effects
The most frequent adverse effects include drowsiness, dizziness, and xerostomia. Central nervous stimulation is more likely to occur in children, and effects can include agitation, insomnia, increased appetite, restlessness, palpitations, muscle spasms, and seizures. Anticholinergic effects can include xerostomia, insomnia, urinary retention, nervousness, mydriasis, xerophthalmia, and blurred vision. Gastrointestinal effects include diarrhea, constipation, and abdominal pain. Quinidine-like anesthetic effects can include sinus tachycardia and cardiac arrhythmias. Blockade of alpha-adrenergic receptors can cause hypotension.
Also see the information regarding use in children from the Centers for Disease Control and Food and Drug Administration previously discussed under the section “Dextromethorphan.”
Generic and Brand Names
Numerous brand and generic products are available without prescription.
Formulations
Products are available as tablets, capsules, gelcaps, orally disintegrating tablets, oral dissolving film, and oral liquids.
EXPECTORANTS
Guaifenesin
Mechanism of Action
Guaifenesin induces expectorant effects by increasing sputum volume and decreasing viscosity presumably by decreasing the surface tension of sputum (Woo, 2008). Ciliary action is improved with the flow of less viscous secretions converting a dry, nonproductive cough to a productive cough (Woo, 2008).
Common Indications
Guaifenesin is indicated for loosening and thinning phlegm associated with coughs from colds and minor upper respiratory tract infections in order to facilitate clearing of bronchial passages and increasing productive cough.
Common Adverse Effects
No adverse effects commonly occur with usual doses.
Also see the information regarding use in children from the Centers for Disease Control and Food and Drug Administration previously discussed under the section “Dextromethorphan.”
Generic and Brand Names
Products containing guaifenesin include Mucinex, Robitussin, as well as numerous other store brands and generics. Guaifenesin is also found in numerous combination products with antihistamines, decongestants, dextromethorphan, and other cough and cold products.
Formulations
Guiafenesin is available as tablets, oral liquids, and granules.
DECONGESTANTS
Oral Decongestants
Mechanism of Action
Oral decongestants reduce nasal blockage by acting directly on α1-receptors, indirectly by displacing norepinephrine from storage vesicles in the nerve terminal, and/or by inhibiting the reuptake of norepinephrine. Pseudoephedrine has direct and/or indirect effects, whereas phenylephrine acts directly on α1-receptors.
Stimulation of postcapillary α1-adrenergic receptors causes vasoconstriction of postcapillary venules in the nasal mucosa (Johnson & Hricik, 1993). These postcapillary venules are capacitance vessels and can accommodate a relatively large amount of blood. Congestion is caused by increased blood volume, which increases the volume of the nasal mucosa. Stimulation of the α1-receptors, which are coupled to G proteins (Gq/11 family), leads to activation of phospholipase Cβ, which results in cleavage of phosphatidylinositol-4,5-bisphosphate into inositol-1,4,5-triphosphate and diacylglycerol (Biaggioni & Roberston, 2009; Hein & Michel, 2007). IP3 promotes the release of intracellular calcium stores, which increases cytoplasmic concentrations of free calcium and activation of calcium-dependent protein kinases to cause vessel contraction. Diacylglycerol activates protein kinase C and modulates the activity of multiple signaling pathways. α1-adrenergic receptors may activate other signaling molecules such as pertussis-sensitive G proteins, Gs family, G12-13 family G proteins, phospholipases A2 via protein kinase C, phospholipase D, and may increase cyclic adenosine monophosphate (Hein & Michel, 2007). These effects shrink swollen nasal mucous membranes to increase nasal airway patency. Sinus drainage is improved and relief from obstructed eustachian ostia may occur.
The only clinically useful oral decongestant available is pseudoephedrine. Phenylephrine is extensively metabolized in the intestinal wall, resulting in low bioavailability; it is unclear if sufficient amounts are absorbed to have a therapeutic effect.
Common Indications
Oral decongestants are indicated for the temporary relief of nasal and sinus congestion due to common cold, allergic rhinitis, or sinusitis, or for relief of eustachian tube congestion. They are also indicted for otalgia prophylaxis (due to air pressure changes) during air travel.
Common Adverse Effects
Common adverse effects from oral decongestant use include headache, increased blood pressure, increased intraocular pressure, insomnia, mydriasis, nervousness, tachycardia, and urinary retention.
Also see the information regarding use in children from the Centers for Disease Control and Food and Drug Administration previously discussed under the section “Dextromethorphan.”
Generic and Brand Names
Oral decongestants are found in many store brand and generic products as well as in numerous combination products with guaifenesin, antihistamines, dextromethorphan, and other cough and cold products.
Formulations
Oral decongestants are available as tablets, oral liquids, and chewable tablets.
Topical Decongestants
Mechanism of Action
Topical decongestants reduce nasal blockage by directly stimulating the α1-receptors in the nose by the same mechanism as described for oral decongestants.
Common Indications
Topical decongestants are indicated for the temporary relief of nasal and sinus congestion due to common cold, allergic rhinitis, or sinusitis, or for the relief of eustachian tube congestion.
Common Adverse Effects
The usual adverse effects related to topical use include sneezing, stinging, transient burning, and ulceration of the nasal mucosa.
Topical decongestants should not be used for longer than 3 days due to the risk of developing rhinitis medicamentosa associated with long-term use.
Generic and Brand Names
Topical decongestants are found in many store brand and generic products.
Formulations
Topical decongestants are available as sprays or drops.
INHALED ANTIBIOTICS
Mechanism of Action
Three antiobiotics for administration by inhalation are commonly available: tobramycin, colistimethate sodium, and aztreonam lysine. Tobramycin is actively transported into the bacterial cell and exerts bacteriocidal activity by irreversibly binding to the 30S bacterial ribosome, which inhibits protein synthesis in susceptible gram-negative bacilli and gram-positive cocci. Binding interrupts messenger RNA action, causing production of abnormal, nonfunctional proteins. Tobramycin must achieve intracellular concentrations in excess of extracellular concentrations in order to be bacteriocidal. Tobramycin has a concentration-dependent killing and a postantibiotic effect against gram-negative aerobic rods and gram-positive organisms.
Colistimethate sodium is bacteriocidal for gram-negative bacteria and acts by binding to bacterial cell membrane phospholipids by displacing calcium and magnesium. This results in increased cell membrane permeability and leakage of cell contents, leading to cell death.
Aztreonam is a synthetic monocyclic beta lactam active against gram-negative aerobic organisms and is stable against most β-lactamases. It acts by binding to penicillin-binding protein-3 of gram-negative rods. The sulfonic acid group promotes acetylation of penicillin-binding protein-3, which is responsible for the development of the septum in cell wall synthesis. Inhibition of the action of penicillin-binding protein-3 prevents cell division and causes the bacterial cell to elongate. Eventual breakage of the cell wall leads to cell lysis and death.
Common Indications
Inhaled tobramycin, inhaled colistimethate sodium, and inhaled aztreonam lysine are indicated for the management of cystic fibrosis patients with Pseudomonas aeruginosa.
Common Adverse Effects
Adverse effects common with intravenous administration of tobramycin are significantly less likely to occur with administration by the inhaled route. Neither renal toxicity nor ototoxicity has been reported during clinical trials (Heijerman et al., 2009), and only dysphonia and mild to moderate tinnitus has occurred more often than with placebo in clinical trials; the latter resolved with drug discontinuation. Bronchospasm may occur; pretreatment with an inhaled β2-agonist may be useful.
Nebulized colistimethate sodium can commonly cause bronchospasm, cough, and throat irritation; inhaled β2-agonist may be administered prophylactically or for treatment of these effects (Heijerman et al., 2009). Irritated oral mucus membranes and Candida superinfection may occur. Colistimethate sodium must be inhaled within 24 hours after reconstitution because when mixed with sterile water, the drug is hydrolyzed into the bases colistin A (polymyxin E1) and colistin B (polymyxin E2) (Heijerman et al., 2009). Polymyxin E1 may cause severe localized airway inflammation and eosinophilic infiltration (Heijerman et al., 2009).
The most common adverse effects with inhaled aztreonam lysine are increased cough, chest tightness, wheezing, pharyngolaryngeal pain, and nasal congestion. As with the other inhaled antibiotics, administration of an inhaled β2-agonist may be used prophylactically or for treatment of bronchial adverse effects.
Generic and Brand Names
Inhaled tobramycin is available as TOBI; colistimethate sodium is available as Coly-Mycin M and generic products; and aztreonam lysine is available as Cayston.
Formulations
Tobramycin is available as a solution for nebulization. Colistimethate sodium and aztreonam lysine are available as a powder for reconstitution for subsequent nebulization.
DELIVERY DEVICES (SEE TABLE 3.1)
MDIs (See Figure 3.1)
MDIs are the standard means to deliver drugs to the lungs due to convenience and efficacy; however, they can be difficult to use correctly. MDIs deliver suspensions or solutions of drug mixed with propellants and other chemicals via a pressurized canister with a metering valve. Technique is extremely important for proper use to ensure delivery to the lower airways; significant hand–lung coordination is required. The majority of patients and health professionals (over 60%) have incorrect technique. Reinstruction is frequently needed because correct use declines over periods as short as 6–10 weeks (De Blaquiere et al., 1989; Dolovich et al., 2000). With appropriate technique, approximately 10–25% of the drug is delivered to the lungs (Dolovich et al., 2000). Breath-actuated devices have further simplified the use of MDIs by significantly lessening the degree of coordination needed. However, these devices provide no advantage to patients with good inhaler technique and cannot be used with spacers and holding chambers, which have advantages of their own.
Table 3.1 Aerosol delivery devices.
Adapted from the National Asthma Education and Prevention Program (2007). Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Heart, Lung, and Blood Institute, publication no. 08-4051.
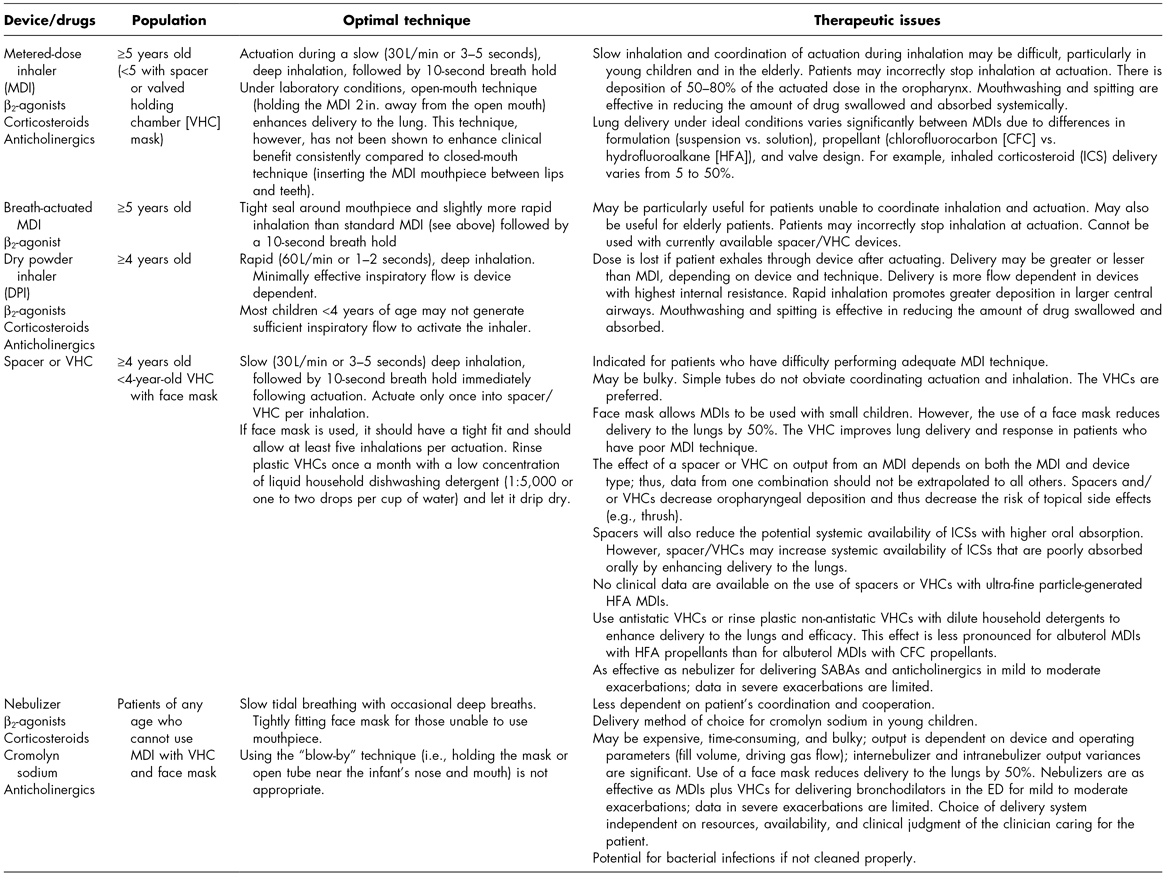
ED, emergency department; SABAs, inhaled short-acting β2-agonists.
Figure 3.1 Metered-dose inhalers (MDIs), nebulizers, and valved holding chambers. From left to right: MDI, breath-actuated MDI, nebulizer cup with face mask, nebulizer cup with mouthpiece, valved holding chamber with mouthpiece, valved holding chamber with face mask.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


