The pediatric airway has many unique features that differ depending on the age of the child (Table 6.1). The larynx is significantly higher in infants and in children. The inferior margin of the cricoid cartilage lies at approximately the level of the second or third cervical vertebra in the infant and descends to the sixth cervical vertebra by puberty (Brown, 2000). The larynx is also located more anterior and superior in the neck in the neonate, allowing approximation of the epiglottis and palate. This allows the neonate to be an obligate nose breather for the first several weeks to months of life. This anatomical situation does allow the healthy neonate the ability to both breath and swallow simultaneously, which is not possible as the larynx descends.
Table 6.1 The pediatric airway is different from the adult airway.
| Development of supporting airway cartilage and small airway muscles is incomplete until school age. |
| The larynx is higher in the child. The cricoid cartilage lies at the level of the third cervical vertebra in the infant and descends to the sixth cervical vertebra at puberty. |
| The thyroid cartilage does not assume its adult configuration until adolescence. |
| The recurrent laryngeal nerve lies just lateral to the trachea. In addition, a pretracheal pad of fat is generally present in infants. |
| The articulation between the head and neck is more mobile in infants and the chin may easily deviate from the midline during surgery. |
| The airways are smaller (narrower and shorter). |
The larynx is composed of cartilage and ligaments. There are three large cartilages and three small cartilages (Becker et al., 1989). The larger cartilages include the thyroid, epiglottis, and cricoid cartilage. The thyroid cartilage forms the main skeleton of the larynx; however, it does not assume its adult configuration until adolescence. The epiglottis cartilage protects the airway from food, liquids, and saliva during swallowing. The cricoid cartilage is located inferior to the thyroid cartilage and is the first ring of the trachea. It is the only rigid circular structure of the airway, which in turn produces the narrowest fixed point in the pediatric airway. In contrast, the narrowest portion of the adult airway is at the level of the vocal cords (Cotton & Willging, 1999). The smaller cartilages include the arytenoid, corniculate, and the cuneiform. The hyoid bone is the only bone in the framework of the larynx. It is a landmark in the neck, a movable horseshoe-shaped bone at the base of the tongue.
The laryngeal muscles are responsible for elevation and depression of the larynx (extrinsic muscles) as well as movement of the vocal cords (intrinsic muscles) (Healy, 1989). The development of all supporting airway cartilage and small airway muscles is incomplete until school age; this may predispose the infant and young child to malacia.
Physiologically, the role of the larynx is multifactorial. It acts as a valve during the swallowing process, closing the airway to prevent aspiration and to allow delivery of food and liquids to the esophagus. During this process, the vocal cords close and the larynx elevates up against the epiglottis. Second, the larynx allows for vocalization. As air is exhaled from the lungs, it travels up through the vocal cords, which vibrate, causing phonation (Brown, 2000). Other aspects of speech are accomplished by structures higher up as the air is exhaled. Articulation is achieved through movement of the soft palate, tongue, and lips. Tone and quality of speech is affected by resonation through the pharynx, sinuses, and nasal passage. The larynx not only serves as an air passage but also as a filter as it clears the respiratory tract of secretions through a voluntary cough. The cough is produced by forcibly closing the glottis.
The recurrent laryngeal nerve and the superior laryngeal nerve are branches of the vagus nerve (cranial nerve X) that supply innervations of the larynx (Becker et al., 1989). The recurrent laryngeal nerve is located just lateral to the trachea and supplies motor innervation to the intrinsic laryngeal muscles, which are responsible for vocal cord mobility. The left branch of the recurrent laryngeal nerve loops under the aortic arch above the pulmonary artery. The trachea is a flexible cylindrical cartilaginous muscular tube extending vertically from the larynx to its bifurcation (carina) into the right and left mainstem bronchi. Its purpose is to transport warmed, filtered, and humidified air to the lungs for respiration. The trachea is composed of 16–20 C-shaped hyaline cartilage “tracheal rings” (Becker et al., 1989). The posterior wall of the trachea (open part of the C) is composed of smooth muscle and connective tissue that allows the diameter of the trachea to change. The posterior trachea abuts the esophagus and the soft posterior wall allows the esophagus to expand during swallowing. The vagus nerve and the sympathetic system innervate the trachea.
It is no surprise that the larynx and trachea are smaller, both narrower and shorter in infants and in children versus adults. The vocal cords in the newborn infant are 6–8 mm long. The posterior glottis transverse length is approximately 4 mm, and the subglottis has a diameter of about 5–7 mm. The tracheal length is 4 cm long (10–13 cm in an adult), with a diameter of 3.6 mm (25 mm in an adult) (Becker et al., 1989). These measurements leave little margin for obstruction.
Pouseuille’s law states that flow within the system is related to the radius of the tube to the fourth power. Resistance is related to the inverse of the radius to the fourth power. One millimeter of tracheal edema will have little effect on the airway of an adult or adolescent; however, it may cause serious airway obstruction and resulting respiratory compromise in an infant or a young child (Table 6.2).
Table 6.2 Tubular fluid dynamics.

ASSESSMENT OF THE CHILD WITH AIRWAY DIFFICULTIES
Obstruction in the airway can present with a vast spectrum of variability from normal breathing with no symptoms to complete obstruction with apnea, loss of consciousness, cyanosis, and potential death. Evaluation of a child with a suspected compromised airway must first and foremost establish the level of urgency of the situation.
History
In an otherwise nonemergent situation, the initial step in the evaluation of airway disease is a careful history. Presence of respiratory symptoms (stridor, work of breathing, history of cyanosis, or apnea) should be ascertained. A careful history of feeding patterns and growth should also be obtained. Infants and children with more severe airway issues frequently present with feeding difficulties and poor weight gain. Quality of voice/cry, presence of birthmarks, history of traumatic birth, and diagnosis of other health problems or syndromes are key aspects of the history. Furthermore, it is important to establish the child’s position of maximum comfort as well as aggravating factors such as excursion, agitation, sleeping, or feeding.
Timing of presentation should be established. It may vary from a prenatal suspicion based on prenatal screening (i.e., tracheal dilation) or prenatal symptoms (i.e., polyhydramnios) to unexpected airway compromise at birth to onset at various postnatal ages. Laryngomalacia, for example, is the most common cause of congenital stridor; however, infants classically present with symptoms at 1 month of age.
Circumstances surrounding the onset of symptoms should be obtained such as recent choking episode, trauma to the head or neck, recent intubation, upper respiratory infection, throat or neck pain, or fever. For example, if signs and symptoms of airway obstruction began following a choking episode, foreign bodies should be considered. Rapidity of progression of symptoms may also be important.
Physical Examination
Physical examination should begin with careful inspection of the patient noting the level of consciousness, position, color, respiratory rate, and effort. General inspection will include identification of the presence or absence of structural anomalies, craniofacial malformations, and presence of lesions (masses or hemangiomas). The examination proceeds with auscultation of the nasal cavity, cheeks, neck, and chest.
Stridor/Stertor
Stridor is the hallmark physical sign of airway obstruction. Stridor is defined as a harsh, vibratory musical sound of varying pitch produced by turbulent airflow through the airway (Healy, 2000). The quality, pitch, and timing of stridor can elicit valuable information regarding the location of the airway obstruction (see Table 6.3). Other key assessments include quality of respirations, work of breathing, as well as the quality of voice or cry.
Table 6.3 Location of airway obstruction based on physical assessment.
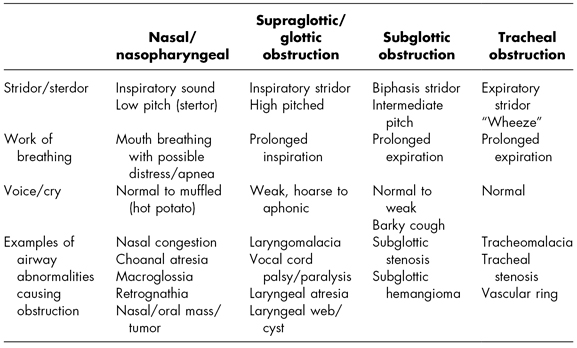
Nasopharyngeal obstruction typically produces an inspiratory low-pitched sound known as stertor. Children with nasopharyngeal obstruction find it easier to breathe through their mouths. Glottic and subglottic obstruction also presents with inspiratory sounds; however, the sound has a high-pitched quality with a prolonged inspiratory phase.
Subglottic obstruction produces biphasic stridor (stridor on both inspiration and expiration). This stridor has an intermediate pitch.
Tracheal obstruction typically produces expiratory stridor, with more of a “wheezy” sound. Supraglottic, subglottic, and tracheal obstructions all tend to produce a prolonged expiratory phase.
The degree (loudness) of stridor or stertor is not a reliable indicator of severity of obstruction (Papsin, Abel, & Leighton, 1999). Stridor can paradoxically become less obvious as obstruction worsens due to diminishing airflow. The degree of stridor is frequently variable based on activity. Feeding and exertion will typically enhance audible stridor.
Retractions
Retractions can be an excellent indicator of the degree of obstruction (increased retractions denote increased obstruction). However, they do not offer much data in respect to location of the obstruction.
Quality of Voice/Cry
The quality of voice and cry varies from normal to muffled with oronasopharyngeal obstruction (oropharyngeal obstruction—hot potato voice; nasopharyngeal obstruction—hyponasal). Laryngeal obstruction naturally affects the voice, ranging from weak to hoarse to aphonia, depending on the location and extent of the obstruction in the larynx. Children with subglottic obstruction will have a normal to weak voice but tend to have a barky (croup-like) cough.
Activity
Many airway lesions will elicit varying symptoms as the child rests, cries, and sleeps. Ideally, it is valuable to assess the child in various positions and states. If possible, attempt further assessment with the child awake or asleep, agitated, during physical exertion, with position changes, and with feedings.
Endoscopy
Endoscopic examination of the patient is the most definitive method of diagnosing a patient with an airway anomaly (Botma, Kishore, Kubba, & Geddes, 2000). Flexible endoscopy allows visualization of the nasal cavity, pharynx, and larynx. Therefore, many etiologies can be ruled in or out based on data from flexible laryngoscopy. It can be performed at the time of initial evaluation, in the office, with the patient awake. This allows for dynamic assessment of the larynx, including vocal cord movement and laryngeal collapse. However, it is not effective in diagnosing lesions below the glottis. It is important to consider that there is a 5% incidence of coexisting lesions in the lower airway when upper lesions are present, and more than 30% have multiple sites of airway abnormalities (Altman, Wetmore, & Mahboubi, 1998). Further endoscopy must also be considered when respiratory symptoms do not match the degree of obstruction identified.
Rigid laryngoscopy and bronchoscopy remain the gold standard for diagnosis of all complex airway lesions. This procedure is accomplished in the operating room with general anesthesia.
Diagnostic Imaging
Radiological evaluation of the pediatric airway is an integral component in the diagnostic and therapeutic management of pediatric airway problems. The diagnostic modality is dependent on the suspected site of obstruction (Zawin, 2000):
- Frontal (anteroposterior) and lateral radiographs are the most common imaging studies used to assess the upper airway. They are useful in providing information regarding the size of the adenoids, epiglottic size and shape, radio-opaque foreign body, retropharyngeal profile, as well as subglottic and tracheal anatomy (Contensin, Gumpert, Gaudemar, Chaussain, & Dupont, 1997).
- Computed tomographic (CT) scan and magnetic resonance imaging (MRI) may be obtained to visualize the airway and the surrounding soft tissue structures.
- Airway fluoroscopy is helpful in offering dynamic information about airway caliber and position during different phases of respiration (Altman et al., 1998).
- Video stoboscopy of the vocal cords is helpful in providing information regarding vocal cord anatomy and movement.
- Barium esophagrams are useful in providing information about the esophageal anatomy and peristalsis, diameter, and mucosal integrity. It can identify an extrinsic compression of the airway, foreign body, strictures, and tracheoesophageal fistulas, and may identify gastroesophageal reflux (however, there is a high percentage of false negatives).
- There are various studies that may be employed to rule out laryngopharangeal reflux including pH probe, impedance probe, barrium swallow, and direct visualization (Karkos, Leong, Apostolidou, & Apostolidis, 2006).
ANOMALIES OF THE LARYNX AND TRACHEA
Laryngomalacia
Laryngomalacia is a common condition in infancy in which the soft, immature, cartilage of the supraglottic larynx collapses inward during inhalation, causing airway obstruction.
Epidemiology
Laryngomalacia is the most common anomaly of the larynx and is the most common cause of stridor in infants (Zoumalan, Maddalozzo, & Holinger, 2007). Males are affected twice as frequently as females. The exact etiology of the disorder is unknown. There are three predominant theories that include immaturity or maldevelopment of the cartilaginous structures, extraesophageal reflux, and immaturity of neuromuscular control.
Histologically, the quality of the cartilage in infants with laryngomalacia is the same as those without. As a result, the pathophysiology of the disorder is also poorly understood. There is some evidence that there may be some genetic predisposition in some cases of laryngomalacia. It is also established that infants with gastroesophageal reflux disease may have more severe diseases (Giannoni, Sulek, Friedman, & Duncan, 1998).
Signs and Symptoms
The infant with laryngomalacia typically presents with inspiratory stridor and feeding difficulties. Stridor is typically accentuated by feeding, agitation, and supine positioning. Conversely, it may improve when the baby is calm, with neck extended, and in a prone position. The onset of stridor is typically in the first several weeks of life. The severity of the stridor may increase with growth over the next few months as air movement becomes more vigorous. It typically slowly resolves by 12–18 months. Feeding difficulties range from prolonged feeding times with pauses or breaks in feeding, irritability during feeds, and postprandial vomiting, to colic to increased stridor during feeding to failure to thrive in infants with more severe laryngomalacia (Giannoni et al., 1998). The child’s cry is typically normal. Respiratory distress, including apnea, cyanosis, retractions, and nasal flaring, is rare. However, some infants will have a more severe form of laryngomalacia, presenting with stridor, severe respiratory distress, and failure to thrive.
Diagnosis
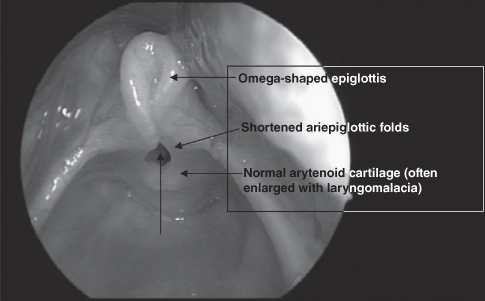
The diagnosis of laryngomalacia is based on the classic history and is confirmed with flexible laryngoscopy (Figure 6.2). The examination will reveal one or more of the characteristic findings of laryngomalacia, including (Richter & Thompson, 2008) the following:
- Omega-shaped epiglottis (elongation and lateral extension) that prolapses posteroinferiorly on inspiration
- Redundant bulky arytenoid mucosa that collapse anteromedially into the airway upon inspiration
- Shortening of the aryepiglottic folds
- Inward collapse of the aryepiglottic folds (cuneiform cartilages) on inspiration
- Anatomy is otherwise normal, including normal structure and function of the vocal cords, and a clear unimpeded airway on expiration.
Management
Most cases of laryngomalacia are managed with careful observation and reassurance. Infants are often evaluated in the office on a monthly basis to monitor respiratory symptoms, weight gain, and feeding tolerance.
The use of antireflux medications, such as a proton pump inhibitor or H2-receptor antagonists, is often helpful in diminishing symptoms, especially in infants with signs and symptoms of gastroesophageal reflux.
Gastroesophageal reflux has been noted in up to 80% of infants with laryngomalacia, many of whom do not exhibit symptoms of reflux (Giannoni et al., 1998). The exposure to acid and pepsin from the reflux of stomach contents can have detrimental effects on the larynx. This may contribute to laryngeal edema, which may exacerbate symptoms of stridor. The use of antireflux medications in infants without clear symptoms of reflux is advocated by some clinicians.
Infants with laryngomalacia who exhibit symptoms of severe respiratory distress and/or failure to thrive will require surgical management. Approximately 10–20% of infants will require surgical intervention to eliminate or bypass the obstruction (Roger, Denoyelle, & Triglia, 1995). Supraglottoplasty is a surgical procedure that involves division of the short aryepiglottic folds. Other revisions to the laryngeal cartilage are sometimes employed, including trimming of the epiglottis, removing the corniculate and cuneiform cartilages, or removing the redundant arytenoid mucosa, all of which may widen the laryngeal inlet (O’Donnell, Murphy, Bew, & Knight, 2007). Tracheotomy may be performed for severe cases not responsive to supraglottoplasty to establish a safe airway.
Complications
Complications are rare as most infants will outgrow the condition within the first 2 years of life. Lower airway lesions may coexist with laryngomalacia and are important to consider and rule out, especially in children with more severe symptoms or atypical history and presentation. Rare complications of severe laryngomalacia include chest deformities, cyanotic attacks, obstructive sleep apnea, pulmonary hypertension, cardiac failure, and failure to thrive (Richter & Thompson, 2008).
Vocal Cord Disorders
There are a variety of vocal cord issues that children may have. This section will outline the most likely types of problems seen: vocal fold immobility (VFI), vocal cord paralysis (VCP), vocal cord dysfunction (VCD), and vocal cord lesions.
Immobility, Paralysis, and Dysfunction
VCP
VCP is the second most common congenital abnormality of the larynx and is responsible for 10% of all congenital airway lesions (Hollinger, Holinger, & Holinger, 1976). VCP occurs secondary to the dysfunction of the nerve supply to the laryngeal muscles, which differs from VFI (often called vocal cord immobility), which is defined as both paralysis and fixation. Additionally, unilateral VCP differs from bilateral VCP in presentation, etiology, and treatment (Chen & Inglis, 2008).
Epidemiology
Bilateral VCP occurs in 30–62% of all cases of VCP, with spontaneous recovery in 48–62% of these cases (Brigger & Hartnick, 2002). Incidence of bilateral VFI is still low overall with reported estimates at 0.75 cases per million births per year (Murty, Shinkwin, & Gibbin, 1994). When looking specifically at reports of unilateral VFI, research is varied. Two reports found in the literature document rates of 8 and 25% (Cavanaugh, 1955; Schild & Holinger, 1980).
Pathophysiology
Children differ from adults in their causes of bilateral VCP. VCP may occur secondary to the stretching and compression of the vagus nerve or the stretching or compression of the recurrent laryngeal nerves (Chen & Inglis, 2008). The causes of nerve injury may be neurological, traumatic, iatrogenic, or idiopathic.
Twenty-five to thirty percent of VCP is from a neurological cause. One of the most common neurological causes is secondary to Arnold–Chiari malformation with associated myelomeningocele and hydrocephalus. It is thought that the vagus nerve becomes stretched and compressed by the protrusion of the cerebellar tonsils, medulla, and brain stem through the foramen magnum (Chen & Inglis, 2008). With this lesion, the infant or child will usually have bilateral paralysis; however, unilateral paralysis has also been reported. Other less common central nervous system lesions include encephalocele, leukodystrophy, hydrocephalus, and cerebral or nuclear dysgenesis. Amyotrophic lateral sclerosis (ALS) may present in the older child and may cause unilateral or bilateral vocal cord involvement. Perinatal hypoxia or cortical stroke is a rare cause (Jong, Kuppersmith, Sulek, & Friedman, 2000). Muscular dystrophies and peripheral neuromuscular disorders have been reported. Examples include myasthenia gravis, myotonic dystrophy, and Charcot–Marie–Tooth disease.
A traumatic cause may be birth trauma. This is secondary to breech presentation, use of forceps for delivery, or intubation, which can cause stretching or compression of one or both recurrent laryngeal nerves of the neck.
The most common iatrogenic cause is secondary to surgery, including patent ductus arteriosis ligation and repair of tracheoesophageal fistula. Paralysis may occur unilaterally or bilaterally. If unilateral, left VCP is more common, secondary to the longer course of the left recurrent laryngeal nerve. Other cardiac surgeries implicated as causes of VCP include those for tetralogy of Fallot and ventricular septal defect. Vascular rings, aortic arch abnormalities, and patent ductus arteriosis surgeries are more typical for laryngeal paralysis (Jong et al., 2000).
Examples of idiopathic etiologies may include infectious, rheumatologic, or mitochondrial disorders (Rubin & Sataloff, 2007). Most of the infectious etiologies of the past, including poliomyelitis and tetanus, are rare today secondary to immunization. Guillian Barre is still a reported cause.
Signs and Symptoms
The most common presenting symptom of bilateral VCP is stridor, which is typically biphasic. Infants will usually have a loud cry. Because the vocal cords are often paralyzed in the paramedian position (abductor paralysis), the voice or cry can be normal. Of note, infants are more likely to present with stridor and dyspnea than with dysphonia or aspiration (Chen & Inglis, 2008). If the cords are paralyzed medially, severe respiratory failure occurs.
Infants with unilateral VCP may present with stridor, or a weak cry (Parikh, 2004). If there is stridor, it will usually be on inspiration, whereas with bilateral paralysis, it is typically biphasic. Symptoms may vary depending on whether the vocal cord is in the paramedian or median position. If the unilateral cord is immobile in the paramedian position, then there is a lack of opposition of the cord and the child may have a weak cry or voice. Over time, the other cord compensates and the child may achieve a more normal voice with time. Feeding difficulties may be present secondary to aspiration or an inability to coordinate the swallow secondary to an incompetent larynx. If there are no feeding or airway problems, the diagnosis may go undetected (Parikh, 2004). Older children and adolescents may present similar to adult patients with a soft or breathy voice disturbances or may have symptoms of dysphagia.
Diagnosis
A full head and neck exam is essential to evaluate for anatomical abnormalities, masses, and/or cranial nerve abnormalities. The child should also be evaluated for any breathing difficulties with particular attention to work of breathing and timing of abnormal respiratory sounds, such as stridor. If suspicion is high for an airway abnormality, then initial consultation of the infant or child with stridor includes airway evaluation with bedside fiber-optic laryngoscopy by an otolaryngologist.
At times, there may be difficulties determining if the VFI is bilateral or unilateral secondary to a crying infant or a limited view of the vocal folds. A confirmatory exam and full evaluation may ultimately be completed by direct laryngoscopy and bronchoscopy in the operating room. The advantage of intraoperative evaluation is the ability to fully evaluate the child for secondary abnormalities such as laryngeal cleft or subglottic stenosis.
Imaging studies such as CT scan or ultrasound are not the standard of care but have been used as diagnostic studies. The utility of the study depends on suspicion of extrinsic lesions or other abnormalities such as central nervous system lesions.
Management
Management differs for bilateral and unilateral VCP. For most cases of bilateral VCP, a tracheostomy will be performed either as a temporary or permanent measure to secure and protect the child’s airway. If the cause is thought to be temporary, the child may remain intubated or under close observation, and tracheostomy may be deferred while awaiting spontaneous recovery.
Depending on the etiology, serial airway exams may be performed to evaluate the infant or child for resolution. Neurological and idiopathic causes have the best chance of spontaneous recovery, which, if it occurs, will generally happen in the first 6 months but can occur up to 11 years after diagnosis (Daya, Hosni, Bejar-Solar, Evans, & Bailey, 2000). For this reason, many otolaryngologists will wait before intervening surgically. Moreover, for infants less than 6 months, treating the underlying cause, such as Arnold–Chiari malformation abnormalities or hydrocephalus, by placement of a ventriculoperitoneal shunt may resolve the paralysis in up to 62% of the cases (Parikh, 2004).
For long-term VCP in children, various surgical techniques such as suture lateralization, arytenoidectomy, cordectomy, and posterior cricoid split with cartilage graft have been described in the literature. Outcome measures include rates of decannulation, which may vary. Some of the risks with surgery include voice disturbances, airway issues, and swallowing dysfunction (Bower, Choi, & Cotton, 1994; Gupta, Mann, & Nagarkar, 1997; Hartnick, Brigger, Willging, Cotton, & Myer, 2003; Inglis et al., 2003; Mathur, Kumar, & Bothra, 2004). There is also a theoretical concern of the effect on laryngeal growth (Parikh, 2004).
In most cases of unilateral VCP, the child will not require any airway intervention. Rarely, neonates with significant airway distress from unilateral paralysis may need a tracheotomy. If the infant or child is experiencing significant aspiration or dysphonia, surgical intervention may be recommended. Most commonly, the vocal fold is medialized by the injection of Teflon, gelfoam, or collagen under endoscopy or by external medialization via thyroplasty. Case numbers are small and studies are limited. Teflon has been reported to have an association with granuloma formulation and is not used as frequently (Parikh, 2004).
Complications
For surgical interventions, a variety of complications can occur, including granuloma formulation, scar formation, ongoing voice or swallowing problems, and pneumonia.
VCD
VCD occurs when there is an abnormal movement of the vocal folds in the absence of other diseases. Another term used to describe this is paradoxic vocal fold motion (PVFM).
Epidemiology
PVFM may occur at any age in children; however, the median reported age of presentation is 14. There is a female preponderance in all age groups (Hicks, Brugman, & Katial, 2008). The true incidence may be higher; however, among healthy, physically active adolescents and young adults, there are reports of incidence that range from 8 to 27% (Morris et al., 1999; Abu-Hasan, Tannous, & Weinberger, 2005; Seear, Wensley, & West, 2005).
Pathophysiology
During a symptomatic period, there is paradoxical symmetrical adduction of the vocal folds toward the midline on inspiration. This causes obstruction at the glottic level. There can also be expiratory obstruction with VCD. At asymptomatic times, the vocal fold motion is normal (Christopher & Morris, 2010).
Precipitating factors have been studied to determine etiologic factors in VCD. Upper airway hyperresponsiveness to a variety of triggers has been considered. While there is no absolute certainty in the triggers of the disease, those with VCD have reported such factors as acid reflux, upper airway infections, allergies, sinusitis, and irritants inhaled from accidental or occupational exposure (Hicks et al., 2008). Exercise may be a trigger and has been reported as a precipitating factor in 18% of people with VCD (Morris, Perkins, & Allan, 2006). Another factor thought to be implicated in VCD is significant psychological stress. Those with VCD may have higher levels of anxiety and more anxiety-related diagnoses (Gavin et al., 1998). The stress of competition for athletes may be noted more frequently as a trigger in exercise-induced VCD (McFadden & Zawadski, 1996). Psychiatric disease has been reported as a factor as well. The most common disorder identified was conversion disorder, followed by depression, Munchausen’s syndrome, obsessive–compulsive disorder, and adjustment disorder (Lacy & McManis, 2004).
Signs and Symptoms
There may be noisy breathing, stridor, wheezing, and/or difficulty breathing, which often occurs on inspiration but can occur on expiration. Cough, chest tightness, throat tightness, and changes in voice have been reported as well, though less commonly (Christopher & Morris, 2010). Symptoms may start and/or end abruptly. Children have presented with asthma-like symptoms and may be diagnosed with exercise-induced asthma. However, they report suboptimal response to bronchodilator therapy.
Diagnosis
Direct laryngoscopy performed at the time of symptoms is the gold standard for the diagnosis of VCD (Morris et al., 2006). Spirometry can be helpful in the diagnosis of a symptomatic child but will not be helpful for children who are asymptomatic at the time of testing. If symptomatic, spirometry will typically reveal a flattened inspiratory loop.
Management
Once the diagnosis is certain, children should be supported and reassured that the condition is benign and self-limiting. Precipitating factors should be identified and avoided. The child may be referred for psychotherapy or psychological counseling and/or speech therapy. Psychotherapy may include biofeedback or hypnotherapy as part of the treatment plan. A speech language pathologist (SLP) can help in the evaluation and treatment of VCD. Therapy usually includes exercises to help abort acute attacks. The SLP can also teach therapeutic exercises to help prevent future episodes.
For exercise-induced VCD, anticholinergic inhalers may be helpful (Doshi & Weinberger, 2006). For acute episodes, there are reports of sedatives and benzodiazepines being used successfully. Bronchodilators, steroids, and other asthma medications show minimal response, though heliox may afford a favorable response. In the event of a very acute onset of symptoms with the appearance of acute respiratory failure, children have been intubated. Often the symptoms quickly resolve and the child is extubated within 24 hours. There are reports of tracheotomy in the literature as well (Christopher & Morris, 2010).
Complications
Complications are secondary to the acute or ongoing treatment of the child before diagnosis, including the overuse of asthma medications, such as corticosteroids. In extreme cases when intubation or tracheotomy is performed, there may be airway or surgical complications to the procedure.
Vocal Cord Masses and Lesions
Most vocal fold lesions are benign in children. This section will briefly discuss two of the more common lesions found in children: vocal cord nodules and recurrent respiratory papillomatosis.
Vocal Cord Nodules
Epidemiology
Vocal nodules are defined as a bilateral symmetric epithelial swelling of the anterior middle third of the true vocal folds (Seear et al., 2005). They can be found in children of all ages. They occur more frequently in females and in those who have high voice demands, including singers and cheerleaders; young adult patients may be teachers.
Signs and Symptoms
Symptoms include chronic hoarseness and episodes of voice loss.
Diagnosis
Diagnosis is typically made by direct laryngoscopy. Ultrasound has recently been reported in the literature as a possible option for diagnosis (Bisetti, 2009).
Management
The main focus of treatment is voice therapy, addressing voice use demands, and minimizing contributing factors. Microsurgical techniques are rarely implemented; however, these may be necessary for suspicious lesions or for those refractory to treatment. If reflux is a contributing factor, a trial of antireflux medication, preferably a proton pump inhibitor, should be considered.
Recurrent Respiratory Papillomatosis
Epidemiology
Recurrent respiratory papillomatosis (RRP) is a chronic disease that affects children and adults. Incidence of juvenile-onset RRP is reported to affect 1 per 100,000 children younger than 18 years per year (Armstrong et al., 2000).
Pathophysiology
It is caused by human papillomavirus (HPV), with strains 6 and 11 being the most common. It is a somewhat rare disease but is the most common cause of laryngeal neoplasm (Altman, 2007). It is thought that a child becomes infected with the virus at the time of birth; however, the virus may remain latent for any number of years. There has been an association between maternal condylomata acuminata and subsequent RRP in an offspring, though some mothers do not have a report of anogenital or cervical warts (Quick, Watts, Krzyzek, & Faras, 1980; Hallden & Majmudar, 1986). Interestingly, there are many infants who are exposed to HPV during the birthing process but never go on to develop the disease, which speaks to other factors, such as immunity, viral load, and/or genetics that may play a role in the acquisition of the disease.
Signs and Symptoms
Children typically present in toddlerhood or as a young adult (Derkay, 2001). Symptoms may include hoarseness/dysphonia, stridor, and/or respiratory distress.
Diagnosis
Diagnosis is typically made by direct laryngoscopy in the office. The warts are typically found in the larynx and are usually on the vocal folds.
Management
The warts may recur frequently, and children often have to undergo repeated laryngoscopy and bronchoscopy with laser excision to maintain their airway and to keep the disease in control. If left unchecked, the warts can grow large enough to obstruct the airway. There is a lot of variability in the course of the disease with some children having procedures monthly and others every few years.
Complications
Distal spread to the trachea is uncommon, but if this occurs, further narrowing of the airway and obstruction of the lung parenchyma can result. It is rare for the papillomas to undergo malignant transformation (Altman, 2007).
Subglottic Stenosis
Subglottis stenosis is a narrowing of the subglottic airway, which is housed in the cricoid cartilage and is described as either congenital or acquired.
Epidemiology
Congenital subglottic stenosis is the third most common congenital anomaly of the airway. Males are affected twice as often as females. Occasionally, it can be associated with congenital abnormalities or syndromes such as Down syndrome (Miller, Gray, Cotton, Myer, & Netterville, 1990). Acquired cases of subglottic stenosis can occur at any age but most commonly occur in the early months of life. It is the most common acquired anomaly of the larynx in children. The true incidence of subglottic stenosis is not known, yet the incidence has been reported between 1 and 8% in neonates requiring endotracheal (ET) intubation for any reason (Cotton & Willging, 1999). The incidence has recently increased with the successful management of low birth weight and babies of earlier gestational age. Conversely, the incidence of the congenital form has remained stable. The population primarily affected by this anomaly is premature infants with immature lungs requiring long-term mechanical ventilation via an endotrachial tube. Subglottic stenosis is the most common abnormality requiring tracheostomy in children less than 1 year of age.
Pathophysiology
The cause of congenital subglottic stenosis is unknown. The infant airway is simply narrower than it should be, causing varying levels of respiratory distress. The severity can vary from very mild narrowing to complete atresia of the larynx. The etiology of the congenital anomaly is due to incomplete recanalization of the laryngotracheal structure in the third month of gestation (Healy, 1989). The anomaly can be divided histopathologically into membranous (circumferential submucosal hypertrophy of the subglottic area) and cartilaginous types (small or malformed cricoid cartilage).
A number of pathogenic processes such as trauma, inflammation, or iatrogenic insults may predispose a child to the development of subglottic stenosis. Endotrachial intubation is the most common factor responsible for acquired subglottic stenosis. The relative size of the ET tube in relation to the child’s larynx, duration of intubation, motion of the tube, and number of repeated intubations have all been implicated as factors influencing its development (Healy, 1989). Other factors implicated in its development include factors affecting wound healing, laryngotracheal reflux, and infection (historically tuberculosis and diphtheria).
Signs and Symptoms
Presentation and severity of symptoms will depend upon the degree of narrowing of the subglottic area. Infants with significant narrowing will have corresponding respiratory distress, including biphasic stridor, croupy cough, retractions, cyanosis, and nasal flaring. Feeding tolerance and appropriate growth can be affected and can be particularly troublesome during respiratory infections. In the most severe cases, it may not be possible for the infant to maintain a safe airway without intubation or tracheostomy.
Infants with mild to moderate subglottic stenosis may be completely asymptomatic with symptoms becoming evident only with an inflammatory process such as an upper respiratory infection. The infant’s symptoms will mirror those of infectious laryngotracheobronchitis (croup); inspiratory or biphasic stridor; and croup, like cough with varying levels of respiratory distress. Subglottic stenosis should be suspected if events are recurrent or if they last for periods longer than 3 days, which is typical of laryngotracheobronchitis. Some children are identified due to difficulty with intubation and extubation. Hoarseness may be present in some infants if the scarring extends superiorly to the vocal cords.
Diagnosis
Definitive diagnosis of subglottic stenosis is made with rigid bronchoscopy (Figure 6.3). The stenosis is evaluated in respect to degree of narrowing, length of narrowing, and to rule out any other airway anomalies. The diagnosis is considered when a 4-mm scope (3 mm in a neonate) or age-appropriate-sized ET tube cannot pass through the subglottic larynx (Healy, 1989). Flexible laryngoscopy is not adequate to evaluate the subglottic area but is useful in ruling out VCP or other glottic and supragrottic disorders and is sometimes used during the same procedure as part of a comprehensive airway evaluation.
Radiological studies such as plain films or CT scans of the neck may be helpful in offering details regarding the length of narrowing, structural information about the larynx, and caliber of the airway (Zawin, 2000). Whenever possible, this information is gathered during the bronchoscopy. However, in severe cases where the scopes are not able to pass through the stenotic area, diagnostic imaging can help plan the ultimate intervention.
Subglottic stenosis is typically classified based on the degree of subglottic narrowing. Myer and Cotton describe a classification system for grading from I to IV (Myer, O’Connor, & Cotton, 1994). This system describes the stenosis as a percentage of the area that is obstructed (Table 6.4).
Table 6.4 Meyer–Cotton staging of subglottic stenosis.
| Staging | Narrowing of subglottic lumen |
| Grade I | Up to 50% |
| Grade II | 51–70% |
| Grade III | 71–99% |
| Grade IV | No detectable lumen |
Management
Management of the child with subglottic stenosis is multifactorial and must be individualized. Infants and children with milder forms of subglottic stenosis (grades I and II) will only require supportive treatment during episodes of upper respiratory infections, and most will completely outgrow the disorder as their airway grows.
There are no medications that will cure subglottic stenosis, yet they may have a supportive role. The use of steroids and/or recemic epinephrine with supplemental oxygen may be indicated during times of increased symptoms with an inflammatory process or with anticipated/recent extubation (Cotton & Willging, 1999). They may be effective in reducing the edema in the airway and may lessen the need for more invasive treatment. Antireflux medications may also be indicated in any child with signs and symptoms of gastroesophageal reflux to reduce the possible increased insult to the airway.
In more severe cases of subglottic stenosis, surgical intervention is warranted to relieve the obstruction. Many factors are taken into consideration when considering surgical repair of subglottic stenosis. The procedure performed is based on the degree of stenosis, length of narrowing, child’s age and weight, presumed etiology, general medical well-being, and coexisting anomalies (Healy, 1989; Hughes & Dunham, 2000).
The cricoid split (anterior laryngotracheal decompression) involves dividing the anterior cricoid ring in an attempt to expand the airway without the need for a tracheostomy. Larygnotracheal reconstruction is a technique that employs cartilaginous grafts (usually from the child’s rib or thyroid cartilage) to stent the subglottis anteriorly and/or posteriorly (Koempel & Cotton, 2008). This reconstruction may be performed as a single stage or multiple stage repair based on the severity of stenosis. Cricotracheal resection with primary anastomosis is advocated in children with severe subglottic stenosis. Tracheostomy is performed to ensure a safe airway, bypassing the level of the obstruction, until a definitive repair or appropriate growth of the airway is achieved. Serial dilations of the subglottic area and/or the use of lasers may be useful as primary treatment or in combination with the procedures previously mentioned.
Endoscopic evaluation and treatments and less invasive procedures may be attempted in some less severe cases. This includes CO2 or KTP laser treatment, dilatation, or balloon laryngoplasty.
Complications
Subglottic stenosis can be life threatening in extreme cases. Furthermore, viral illness and other upper respiratory infections may cause further narrowing of the airway, resulting in significant respiratory distress.
Laryngeal Webs
Laryngeal webs are a banding of tissue across the larynx resulting in varying degrees of obstruction.
Epidemiology
The incidence of laryngeal webs is 1/10,000 births. Although laryngeal webs may be an isolated finding, it is commonly associated with other congenital defects, the most prevalent being chromosomal and cardiovascular (McDonald-McGinn, Zachai, & Goldmuntz, 2002). There is a known association of congenital laryngeal webs with velocardiofacial syndrome (chromosome 22q11.2 deletion, also known as DiGeorge syndrome) (Dyce, McDonald-McGinn, & Kirschner, 2002). Approximately a third of children with laryngeal webs will have an additional anomaly in the respiratory tract.
Pathophysiology
Similar to congenital subglottic stenosis, laryngeal webs are the result of failure of laryngeal recanalization in the third gestational month. This can result in either complete occlusion of the larynx by mucosal and submucosal tissue or partial occlusion by a thin, membranous web. Webs may occur in a variety of locations in the larynx, including most commonly the anterior vocal folds, posterior interarytenoid, or in the subglottic or supraglottic areas. Although typically congenital, they may be acquired secondary to a surgical procedure, intubation, or infection (corynebacterium diphtheria or Bacillus cereus) (Desuter, Veyckemans, & Clement de Clety, 2004).
Signs and Symptoms
Symptoms depend on the location and degree of webbing that is present. Infants typically present with a weak or absent cry (Nicollas & Triglia, 2008). They may have varying levels of stridor and respiratory distress, including retractions, nasal flaring, and cyanosis. These symptoms are not positional.
Diagnosis
Fiber-optic laryngoscopy is useful in identifying a laryngeal web. Rigid laryngoscopy and bronchoscopy is employed for further evaluation of the web and the remaining airway. It assesses the web’s location, thickness, and horizontal and vertical extent (Nicollas & Triglia, 2008). The remainder of the airway must also be inspected to rule out other anomalies. Genetic testing may be considered to rule out velocardiofacial syndrome in patients with anterior webs.
Management
Treatment may vary from lysis, excision, dilation, cryotherapy, and CO2 laser to obliterate the web (Nicollas & Triglia, 2008). Surgical goals are to resolve airway obstruction and to ensure quality vocal function. Thicker webs may require stenting with temporary protection of the airway with tracheostomy. Revision procedures are sometimes indicated in more complicated or posterior webs.
Subglottic Hemangioma
Subglottic hemangiomas are benign vascular tumors found in the subglottis.
Epidemiology
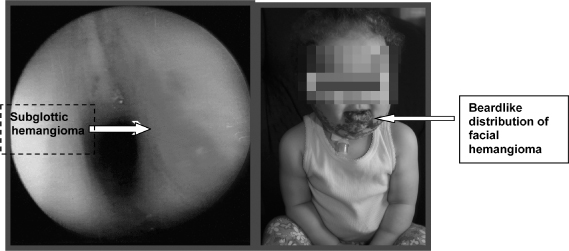
Hemangioma of infancy is the most common tumor of childhood. There is a 10% incidence in infants. It is seen in all racial groups but is more common in Caucasians. Children with beardlike distribution of hemangioma have a higher incidence of airway involvement (Orlow, Isakoff, & Blei, 1997) (Figure 6.4). Fifty percent of patients with subglottic hemangioma have a concomitant cutaneous lesion, yet only 1–2% of patients with a cuteanous hemangioma also have a subglottic lesion. Laryngeal lesions comprise 1.5% of all congenital anomalies of the larynx (O-Lee & Messner, 2008). Females are affected twice as much as males. Hemangiomas of the tongue, choana, and palate may also cause substantial airway obstruction.
Figure 6.4 Subglottic hemangioma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree