CHAPTER 110 For more information on the weapons discussed here, or for information on other potential weapons, you can consult the online resources listed in Table 110–1. TABLE 110–1 Online Resources for Information on Biologic, Radiologic, and Chemical Terrorism • www.bt.cdc.gov—Bioterrorism information from the Centers for Disease Control and Prevention (CDC) • www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/default.htm—Bioterrorism information from the U.S. Food and Drug Administration (FDA) • www.bioterrorism.slu.edu—Bioterrorism information from the Center for the Study of Bioterrorism at the Saint Louis University School of Public Health • www.idsociety.org/bt/toc.htm—Bioterrorism information from the Infectious Disease Society of America • www.upmc-biosecurity.org—Information on biologic weapons from the Center for Biosecurity, an independent, nonprofit organization associated with the University of Pittsburgh Medical Center • Jama.ama-assn.org/cgi/collection/bioterrorism—Articles on bioterrorism from JAMA • www.who.int/ionizing_radiation/a_e/terrorism/en/—Information on nuclear weapons and dirty bombs from the World Health Organization Given the rapid course that inhalational anthrax follows, early therapy with antibiotics is essential. Any delay can reduce the chance of survival. Initial IV therapy is preferred to initial oral therapy. However, if there are mass casualties, IV therapy may be impossible, owing to limited supplies and personnel. Ideally, treatment should start with IV ciprofloxacin or IV doxycycline. Because the strain of B. anthracis may be resistant to these drugs, one or two other IV antibiotics should be included. When clinically appropriate, the patient can be switched to oral ciprofloxacin or doxycycline, without additional antibiotics. The duration of treatment—IV plus oral—is 60 days. Specific regimens for adults, children, and pregnant women are presented in Table 110–2 (for limited casualty settings) and Table 110–3 (for mass casualty settings). TABLE 110–2 Therapy of Inhalational Anthrax in the Limited Casualty Setting Data from Inglesby TV, et al: Anthrax as a biological weapon, 2002: Updated recommendations for management. JAMA 287:2236–2252, 2002. TABLE 110–3 Therapy of Inhalational Anthrax in the Mass Casualty Setting Data from Inglesby TV, et al: Anthrax as a biological weapon, 2002: Updated recommendations for management. JAMA 287:2236–2252, 2002. Cutaneous anthrax is treated with oral antibiotics. The preferred drugs are ciprofloxacin and doxycycline. Dosages for adults, children, and pregnant women are the same as those given in Table 110–2 for follow-up oral therapy of inhalational anthrax. Duration of treatment is 60 days. It should be noted that treatment is unlikely to prevent cutaneous lesions, but will prevent systemic complications. To prevent infection following exposure to aerosolized anthrax spores, the Centers for Disease Control and Prevention (CDC) recommends treatment with an oral antibiotic plus anthrax vaccine. Antibiotic regimens are the same ones employed for treating inhalational anthrax in a mass casualty setting (see Table 110–3). Dosing should start immediately and continue for at least 60 days. New recombinant vaccines are in development. However, when they will be available is uncertain.
Potential weapons of biologic, radiologic, and chemical terrorism

Bacteria and viruses
Bacillus anthracis (anthrax)
Treatment of established infection
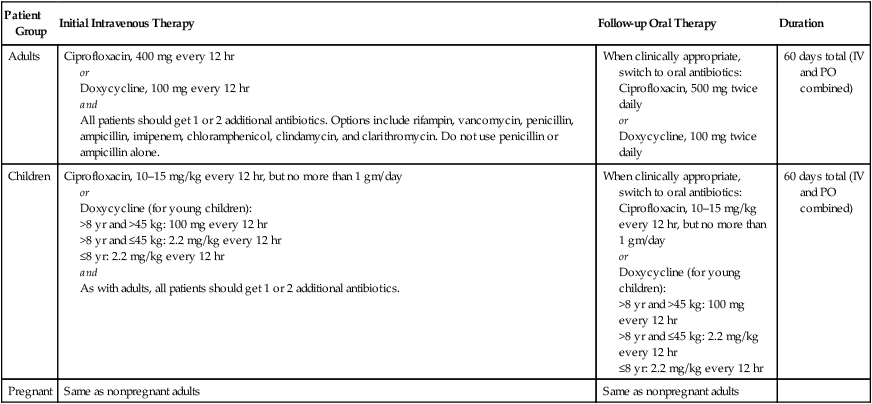
Inhalational anthrax.

Patient Group
Initial Intravenous Therapy
Follow-up Oral Therapy
Duration
Adults
Ciprofloxacin, 400 mg every 12 hr
or
Doxycycline, 100 mg every 12 hr
and
All patients should get 1 or 2 additional antibiotics. Options include rifampin, vancomycin, penicillin, ampicillin, imipenem, chloramphenicol, clindamycin, and clarithromycin. Do not use penicillin or ampicillin alone.
When clinically appropriate, switch to oral antibiotics:
Ciprofloxacin, 500 mg twice daily
or
Doxycycline, 100 mg twice daily
60 days total (IV and PO combined)
Children
Ciprofloxacin, 10–15 mg/kg every 12 hr, but no more than 1 gm/day
or
Doxycycline (for young children):
>8 yr and >45 kg: 100 mg every 12 hr
>8 yr and ≤45 kg: 2.2 mg/kg every 12 hr
≤8 yr: 2.2 mg/kg every 12 hr
and
As with adults, all patients should get 1 or 2 additional antibiotics.
When clinically appropriate, switch to oral antibiotics:
Ciprofloxacin, 10–15 mg/kg every 12 hr, but no more than 1 gm/day
or
Doxycycline (for young children):
>8 yr and >45 kg: 100 mg every 12 hr
>8 yr and ≤45 kg: 2.2 mg/kg every 12 hr
≤8 yr: 2.2 mg/kg every 12 hr
60 days total (IV and PO combined)
Pregnant
Same as nonpregnant adults
Same as nonpregnant adults

Patient Group
Preferred Initial Oral Therapy
Alternative Oral Therapy (if Strain Is Proved Susceptible)
Duration
Adults
Ciprofloxacin, 500 mg every 12 hr
Doxycycline, 100 mg every 12 hr
Amoxicillin, 500 mg every 8 hr
60 days
Children
Ciprofloxacin, 10–15 mg every 12 hr, but no more than 1 gm/day
≥20 kg: amoxicillin, 500 mg every 8 hr
<20 kg: amoxicillin, 13.3 mg/kg every 8 hr
60 days
Pregnant
Ciprofloxacin, 500 mg every 12 hr
Amoxicillin, 500 mg every 8 hr
60 days
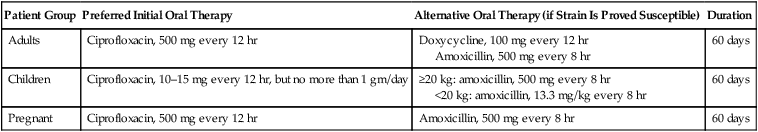
Cutaneous anthrax.
Postexposure prophylaxis: antibiotics plus vaccination
Variola virus (smallpox)
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Potential weapons of biologic, radiologic, and chemical terrorism
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access
