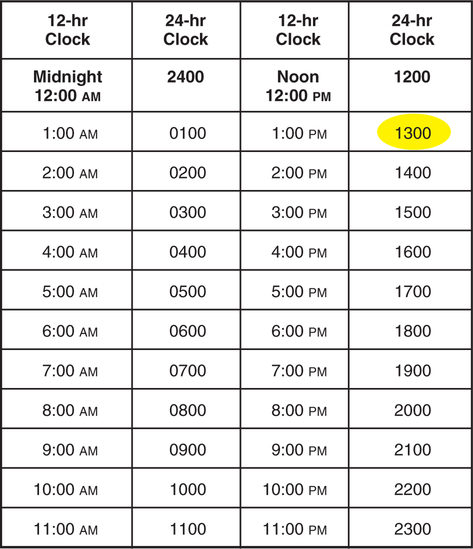
• Interpret medication orders and labels. • Identify abbreviations that cannot be used for handwritten medical records. • Identify abbreviations that can lead to medication errors. • Utilize TJC and ISMP medication-related recommendations • Identify forms of medications. • Read and write time using the 24-hour clock. • Describe the data from the order and label that must be entered in all medication calculations. • Interpret Medication Administration Records (MARs). • Describe medication-related nurse actions that may lead to medication errors. 1. What is the difference between a trade-name product and a generic product and why must the nurse know the difference? _________________________________________________________________________ 2. What are some examples of drug forms? _________________________________________________________________________ 3. How may unit-dose packaging help prevent a medication error? _________________________________________________________________________ 4. What is the abbreviation for the daily medication record maintained by the nurses? _________________________________________________________________________ Medications for institutional use are stored in locked cabinets, carts, and drawers (Figure 4-1). Bar codes are increasingly used to reduce medication errors. The pharmacy enters the patients’ drug information, records, and medication orders into a computer. Each dose is bar-coded in the pharmacy. The nurse uses a hand-held scanner to scan the drug and the patient’s wrist bracelet (Figure 4-2). The computer checks that it is the right medication for the right patient. Unit-dose medications supplied by the pharmacy also reduce medication errors. Medications are supplied in a variety of solid and liquid forms, including granules, tablets, capsules, suppositories, and various liquid preparations. The physician’s order must specify the form of the medication. A single medication may be prepared in several forms and marketed and packaged in several single and/or multidose sizes. Multidose sizes are convenient for pharmacy and home use (Figure 4-3). At most clinical facilities, the pharmacy packages smaller single-serving-size amounts from the multidose containers to reduce medication errors (Figure 4-4). Table 4-1 lists solid medication abbreviations, terms, and forms. Since the nurse must be able to interpret the abbreviations and terms, the contents of Table 4-1 should be memorized. TABLE 4-1 *Does not mean long-acting or extended-release. However, a DS pill probably will be given less frequently than a “regular” counterpart. 1. How is DS different from medications marked XL, XR, CD, and LA? _________________________________________________________________________ Liquid drug forms are administered using specially calibrated equipment: cups; teaspoons; needles attached to tubing; syringes with needles; needle-less syringes; droppers for the mouth, eye, or ear; or tubes for the stomach and intestine. See Figures 4-5 and 4-6 for some examples of oral liquid medication equipment. Doses of oral liquid medications such as milk of magnesia (MOM) can be supplied in small single-dose packages or larger multidose bottles. As stated in Chapter 3, 5 mL and 15 mL are the equivalents of 1 calibrated teaspoon and 1 calibrated tablespoon, respectively. The manufacturer attempts to provide the usual drug dose for the target population within those two measurements because they are reasonable volumes to swallow. Abbreviations appear in most patients’ orders and medication records. They may be handwritten or printed. Memorize the abbreviations in Table 4-2. TABLE 4-2 1. Which of the oral liquid drug forms contains alcohol? _________________________________________________________________________ 2. Which of the liquid drug forms must be gently and thoroughly mixed before administration? _________________________________________________________________________ Medication routes can be divided into two types: Nonparenteral routes through which medications are delivered include the following: Several abbreviations are used in medication orders to describe specific nonparenteral routes of administration (Table 4-3). Some of these abbreviations are derived from Latin and Greek. The abbreviations must be learned even though the trend is to write more of them out in English to avoid misinterpretation. TABLE 4-3 Nonparenteral Medication Routes 1. What is the difference between NPO and PO? _________________________________________________________________________ 2. What is the difference between the buccal and sublingual routes? _________________________________________________________________________ 1. What cue will help you remember that PO means “by mouth”? _________________________________________________________________________ 2. How will you distinguish subl and subcut? _________________________________________________________________________ 3. How will you distinguish GT and NGT? _________________________________________________________________________ The routes listed in Table 4-4 are for parenteral, or injectable, medications. Parenteral medications are administered under the skin into soft tissue, muscle, vein, or spinal cord. Memorize the abbreviations and terms in the table. TABLE 4-4 *Do not confuse ID route with the word “identify.” 4. Why should the route for rectal medications be spelled out rather than abbreviated with the letter R? _________________________________________________________________________ 5. How will you distinguish IV, IVP, and IVPB? _________________________________________________________________________ 6. What is the abbreviation for the intramuscular route? _________________________________________________________________________ 7. What is the abbreviation for the intradermal route? _________________________________________________________________________ 8. What is the abbreviation for metered dose inhaler? _________________________________________________________________________ 9. What kind of solution is PN? _________________________________________________________________________ Table 4-5 lists abbreviations for terms that denote the frequency and times of medication administration. Memorize the abbreviations in the table. TABLE 4-5 Refer to the TJC “Do Not Use” list on p. 101. Refer to the ISMP List of Error-Prone Abbreviations, Symbols, and Dose Designations on pp. 102-103. 1. How will you remember to distinguish the abbreviations for before and after meals? _________________________________________________________________________ 1. What is the error on the TJC list, Table 4-6 that can occur with abbreviations beginning with a “Q”? TABLE 4-6 The Joint Commission Official “Do Not Use” List Copyright The Joint Commission, 2010. Reprinted with permission. †Exception: A “trailing zero” may be used only where required to demonstrate the level of precision of the value being reported, such as for laboratory results, imaging studies that report size of lesions, or catheter/tube sizes. It may not be used in medication orders or other medication-related documentation. _________________________________________________________________________ 2. What is the error that can occur with the abbreviation for International Unit? _________________________________________________________________________ 3. To which kind of specific orders and documentation must the TJC official “Do Not Use” list apply? _________________________________________________________________________ 4. What does the ISMP List, Table 4-7, recommend about writing of drug names and doses? Numerical doses and unit of measure? TABLE 4-7 ISMP’s List of Error-Prone Abbreviations, Symbols, and Dose Designations
Patient Records, Medication Orders, and Labels
Introduction
Medication Storage and Security

![]() Refer to Essential Vocabulary in this chapter and Appendix B for a list of high-alert medications.
Refer to Essential Vocabulary in this chapter and Appendix B for a list of high-alert medications.
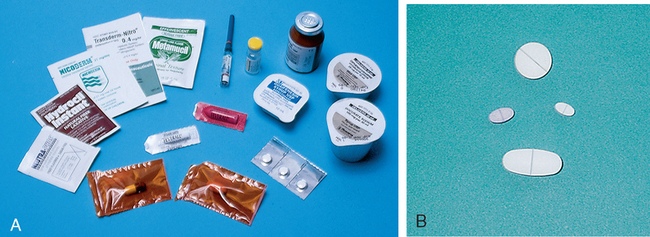
Medication Forms and Packaging
Solid Drug Forms
Abbreviation or Term
Description
cap: capsule
Medication covered in hard or soft gelatin. They are supplied in various sizes. The entire contents may be sprinkled in food such as applesauce or a liquid if the physician so specifies. Capsules should never be cut or divided into partial amounts.
caplet
Smooth, lightly coated, small oval tablet. The name is derived from capsule and tablet. It may or may not be scored.
compound
Medication consisting of a combination of two or more drugs. Each ingredient may be available in one or more strengths. The order will specify the number of tablets. If there is more than one strength, the order will specify the strength.
enteric-coated tablet (Always write out.)
Tablet containing potentially irritating substances and covered with a coating that delays absorption until it reaches the intestine. This protects the oral, esophageal, and gastric mucosa. Should not be crushed, cut, or chewed. Enteric should be written out to avoid misunderstanding.
gelcap, soft-gel
Capsule cover made of a soft gelatin for ease of swallowing.
Oral dissolving tablet (ODT)
Tablet that dissolves in the mouth and does not need to be taken with water.
powders and granules
Pulverized fragments of solid medication, to be measured and sprinkled in a liquid or a food such as applesauce or cereal.
scored tablet
Tablets scored with a dividing line that may be cut in half.
supp: suppository
Medication distributed in a glycerin-based vehicle for insertion into the rectum, vagina, or urethra and absorbed systemically.
tab: tablet
Medication combined with a powder compressed into small round and other shapes.
ung: ointment
Medication contained within a semisolid petroleum or cream base.
CD: controlled-dose (sustained action)
Terms reflecting the use of various processing methods to extend or delay the release and absorption of the medication. They need to be differentiated from a regular form of the same medicine.
DS: double-strength*
A regular medication may be ordered, for example, every 4 hours. An XR version may be given only every 12 or 24 hours. Some medications, such as Celexa, are marketed in both SR and XL forms.
LA: long-acting
SR: slow-release
XL: extra-long-acting
XR: extended-release
![]() A single medicine may be available as a tablet, a capsule, a gel cap, and an enteric-coated tablet. When there is a choice, the prescriber offers the one that is best for the patient.
A single medicine may be available as a tablet, a capsule, a gel cap, and an enteric-coated tablet. When there is a choice, the prescriber offers the one that is best for the patient.
![]() Never crush gelcaps, enteric-coated or other long-acting or slow release medications. Only scored tablets should be cut.
Never crush gelcaps, enteric-coated or other long-acting or slow release medications. Only scored tablets should be cut.
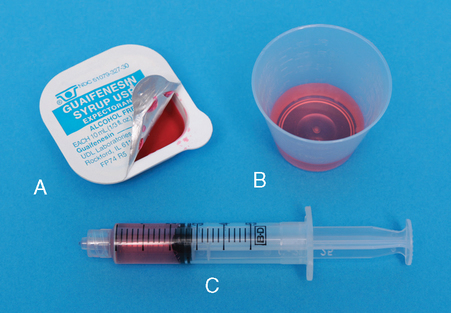
Liquid Drug Forms
Abbreviation: Term
Form
aq: aqueous
Medication supplied in a water-based solution
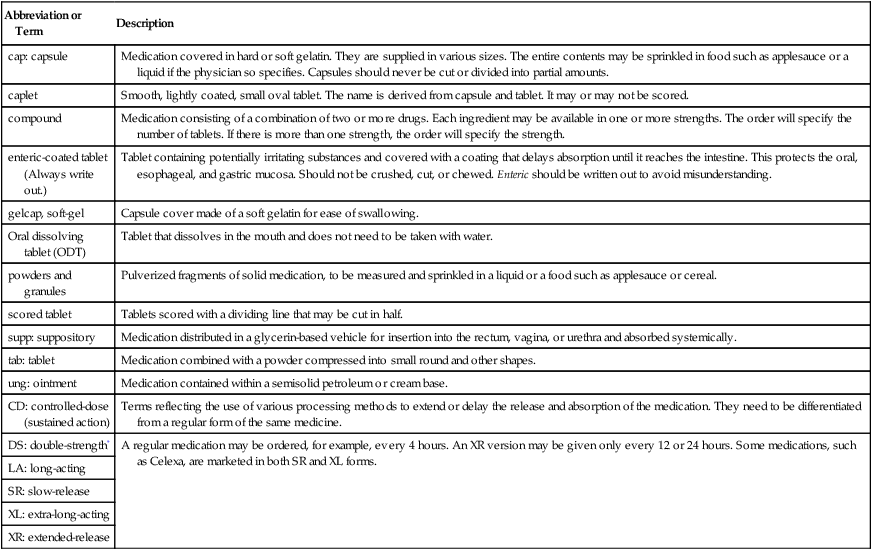
elix: elixir
Liquid medication sweetened with alcohol, e.g., elixir terpin hydrate (ETH), a cough medicine
emul: emulsion
A mixture of two liquids, such as oil and water, that normally do not mix
fld: fluid
Of liquid composition
gtt
gtt is an old abbreviation derived from Latin meaning drop. Recognize it but do not write it. Write out “drop(s).”
mixt: mixture
Compound medicine consisting of more than one liquid medication
sol: solution
Water-based liquid medication
susp: suspension
Solid particles mixed in liquid that must be gently but thoroughly mixed immediately before administration; should not be shaken vigorously
![]() Manufacturer-supplied equipment provides precise dose measurement.
Manufacturer-supplied equipment provides precise dose measurement.
![]() Liquid forms such as elixirs and suspensions cannot be distinguished without reading the label.
Liquid forms such as elixirs and suspensions cannot be distinguished without reading the label.
Medication Routes
![]() The nurse may not substitute a different route for the one ordered. Only the prescriber can write and change the medication order.
The nurse may not substitute a different route for the one ordered. Only the prescriber can write and change the medication order.
Abbreviation or Term
Route
Ear and eye (write out)
Right ear or eye
Ear and eye (write out)
Left ear or eye
Ear and eye (write out)
Both ears or eyes
buccal (bucc)
To be dissolved in the cheek, not swallowed
enteric (write out)
Administered through a tube or port to the small intestine
GT
Gastrostomy tube; given through a tube or port directly to the stomach
MDI
Metered dose inhaler
NG (T)
Nasogastric; given through a tube inserted in the nose to the stomach
NPO
Nothing by mouth
PO
Given by mouth
Rectal
Write “per rectum”; do not write “PR”
SL, subl
Sublingual, meaning “under the tongue”; to be dissolved, not swallowed
Top
Topical, meaning “applied to the skin” (e.g., ointments and lotions)
Vag
Given per vagina
![]() Refer to pp. 102-103 for the ISMP list of error-prone abbreviations and symbols.
Refer to pp. 102-103 for the ISMP list of error-prone abbreviations and symbols.
Abbreviation or Term
Route
epidural
Injected into the epidural space, usually in the lumbar region
hypo
Hypodermic; injected under the skin; refers to subcutaneous and intramuscular routes
ID
Intradermal; given under the skin in the layer just below epidermis (e.g., skin test)*
IM
Intramuscular, intramuscularly; given into the muscle layer, usually the gluteal muscles, the thigh, or the deltoid
intrathecal
Given into the spinal canal
IV
Intravenous, intravenously; given into vein
IVPB
Intravenous piggyback; given into vein via a small container attached to an established intravenous line.
subcut†
Subcutaneous, subcutaneously; given beneath the skin, usually into the fat layer of abdomen, upper arm, or thigh
PN
Parenteral nutrition; nutritional feedings per intravenous line into a large vein
Frequency and Times of Medication
Abbreviation or Term
Meaning
ā or a
Before
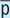
After
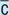
With (e.g., with meals)
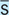
Without
ac
Before meals
pc
After meals
ad lib
Give as desired; pertains to fluids and activity, not to medication
bid
Two times a day
tid
Three times a day
qid
Four times a day
on call
Give the medication when the x-ray department or the operating room (OR) calls for the patient
prn
As needed
q
Each, every
qh
Every hour
q2h
Every 2 hours
q4h
Every 4 hours
q6h
Every 6 hours
q8h
Every 8 hours
q12h
Every 12 hours
qhs
Every night at bedtime
stat
Immediately and only one dose
![]() “Give with food” and “Give after meals” are usually orders for medications that irritate the gastric mucosa.
“Give with food” and “Give after meals” are usually orders for medications that irritate the gastric mucosa.
![]() “Give 1 hour before meals” and “Give 2 hours after meals” are orders for medications that have reduced absorption if given with food.
“Give 1 hour before meals” and “Give 2 hours after meals” are orders for medications that have reduced absorption if given with food.
Do Not Use
Potential Problem
Use Instead
U (unit)
Mistaken for “0” (zero), the number “4” (four) or “cc”
Write “unit”
IU (International Unit)
Mistaken for IV (intravenous) or the number 10 (ten)
Write “International Unit”
Q.D., QD, q.d., qd (daily)
Mistaken for each other
Write “daily”
Q.O.D., QOD, q.o.d., qod (every other day)
Period after the Q mistaken for “I” and the “O” mistaken for “I”
Write “every other day”
Trailing zero (X.0 mg)†
Decimal point is missed
Write X mg
Lack of leading zero (.X mg)
Write 0.X mg
MS
Can mean morphine sulfate or magnesium sulfate
Write “morphine sulfate”
MSO4 and MgSO4
Confused for one another
Write “magnesium sulfate”
Additional Abbreviations, Acronyms and Symbols (For possible future inclusion in the Official “Do Not Use” List)
Do Not Use
Potential Problem
Use Instead
> (greater than)
Misinterpreted as the number “7” (seven) or the letter “L”
Write “greater than”
< (less than)
Confused for one another
Write “less than”
Abbreviations for drug names
Misinterpreted due to similar abbreviations for multiple drugs
Write drug names in full
Apothecary units
Unfamiliar to many practitioners
Use metric units
Confused with metric units
@
Mistaken for the number “2” (two)
Write “at”
cc
Mistaken for U (units) when poorly written
Write “mL” or “ml” or “milliliters” (“mL” is preferred)
μg
Mistaken for mg (milligrams) resulting in one thousand-fold overdose
Write “mcg” or “micrograms”
Dose Designations and Other Information
Intended Meaning
Misinterpretation
Correction
Drug name and dose run together (especially problematic for drug names that end in “I” such as Inderal40 mg; Tegretol300 mg)
Inderal 40 mg
Mistaken as Inderal 140 mg
Place adequate space between the drug name, dose, and unit of measure
Tegretol 300 mg
Mistaken as Tegretol 1300 mg
Numerical dose and unit of measure run together (e.g., 10mg, 100mL)
10 mg
The “m” is sometimes mistaken as a zero or two zeros, risking a 10- to 100-fold overdose
Place adequate space between the dose and unit of measure
100 mL
Abbreviations such as mg. or mL.with a period following the abbreviation
mg
The period is unnecessary and could be mistaken as the number 1 if written poorly
Use mg, mL, etc.without a terminal period
mL
Large doses without properly placed commas (e.g., 100000 units; 1000000 units)
100,000 units
100000 has been mistaken as 10,000 or 1,000,000; 1000000 has been mistaken as 100,000
Use commas for dosing units at or above 1,000, or use words such as 100 “thousand” or 1 “million” to improve readability
1,000,000 units
Drug Name Abbreviations
Intended Meaning
Misinterpretation
Correction
ARA A
vidarabine
Mistaken as cytarabine (ARA C)
Use complete drug name
AZT
zidovudine (Retrovir)
Mistaken as azathioprine or aztreonam
Use complete drug name
CPZ
Compazine (prochlorperazine)
Mistaken as chlorpromazine
Use complete drug name
DPT
Demerol-Phenergan- Thorazine
Mistaken as diphtheria- pertussis-tetanus (vaccine)
Use complete drug name
DTO
Diluted tincture of opium, or deodorized tincture of opium (Paregoric)
Mistaken as tincture of opium
Use complete drug name
HCI
hydrochloric acid or hydrochloride
Mistaken as potassium chloride (The “H” is misinterpreted as “K”)
Use complete drug name unless expressed as a salt of a drug
HCT
hydrocortisone
Mistaken as hydrochlorothiazide
Use complete drug name
HCTZ
hydrochlorothiazide
Mistaken as hydrocortisone (seen as HCT250 mg)
Use complete drug name
MgSO4*
magnesium sulfate
Mistaken as morphine sulfate
Use complete drug name
MS, MSO4*
morphine sulfate
Mistaken as magnesium sulfate
Use complete drug name
MTX
methotrexate
Mistaken as mitoxantrone
Use complete drug name
PCA
procainamide
Mistaken as patient controlled analgesia
Use complete drug name
PTU
propylthiouracil
Mistaken as mercaptopurine
Use complete drug name
T3
Tylenol with codeine No.3
Mistaken as liothyronine
Use complete drug name
TAC
triamcinolone
Mistaken as tetracaine, Adrenalin, cocaine
Use complete drug name
TNK
TNKase
Mistaken as “TPA”
Use complete drug name
ZnSO4
zinc sulfate
Mistaken as morphine sulfate
Use complete drug name
Stemmed Drug Names
Intended Meaning
Misinterpretation
Correction
“Nitro” drip
nitroglycerin infusion
Mistaken as sodium nitroprus-side infusion
Use complete drug name
“Norflox”
norfloxacin
Mistaken as Norflex
Use complete drug name
“IV Vanc”
intravenous vancomycin
Mistaken as Invanz
Use complete drug name
Symbols
Intended Meaning
Misinterpretation
Correction

Dram
Symbol for dram mistaken as “3”
Use the metric system
Minim
Symbol for minim mistaken as “mL”
Use the metric system
x3d
For three days
Mistaken as “3 doses”
Use “for three days”
and
Greater than and less than
Mistaken as opposite of intended; mistakenly use incorrect symbol; “ 10” mistaken as “40”
Use “greater than” or “less than”
/(slash mark)
Separates two doses or indicates “per”
Mistaken as the number 1 (e.g., “25 units/10 units” misread as “25 units and 110” units)
Use “per” rather than a slash mark to separate doses
@
At
Mistaken as “2”
Use “at”
&
And
Mistaken as “2”
Use “and”
+
Plus or and
Mistaken as “4”
Use “and”
°
Hour
Mistaken as a zero (e.g., q2º seen as q 20)
Use “hr, ”“h,” or “hour” ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Patient Records, Medication Orders, and Labels
Get Clinical Tree app for offline access