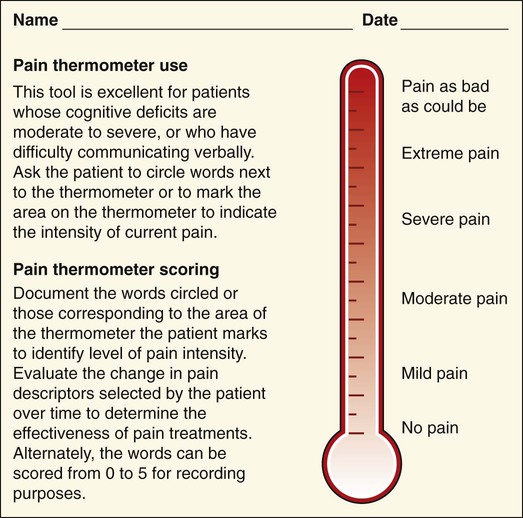
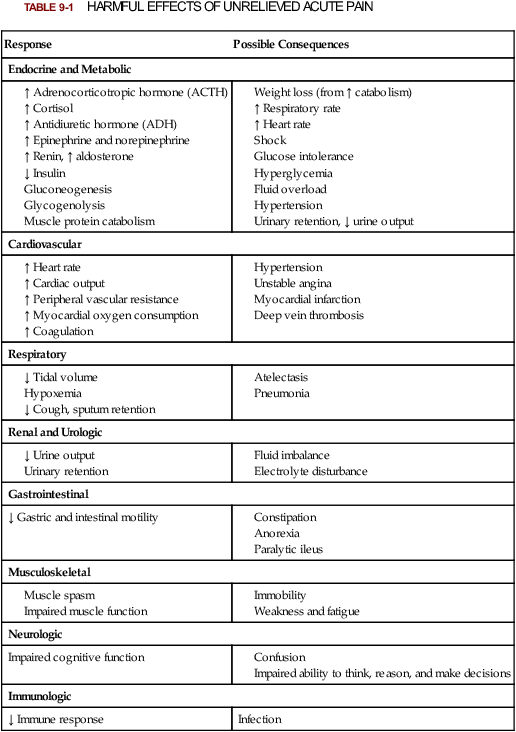
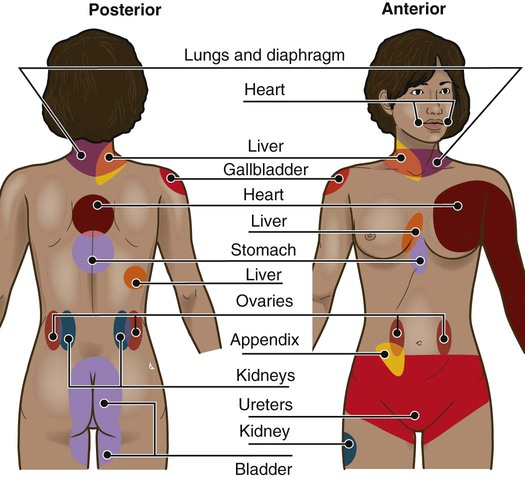
Lindsay L. Kindler and Rosemary C. Polomano 2. Describe the neural mechanisms of pain and pain modulation. 3. Differentiate between nociceptive and neuropathic types of pain. 4. Explain the physical and psychologic effects of unrelieved pain. 5. Interpret the subjective and objective data that are obtained from a comprehensive pain assessment. 6. Describe effective interdisciplinary pain management techniques. 7. Describe drug and nondrug methods of pain relief. 8. Explain your role and responsibility in pain management. 9. Discuss ethical and legal issues related to pain and pain management. 10. Evaluate the influence of one’s own knowledge, beliefs, and attitudes about pain assessment and management. Every year, millions of people suffer from pain. Annually in the United States, at least 25 million people experience acute pain as a result of injury or surgery.1 Common chronic pain conditions such as arthritis, migraine headache, and back pain affect approximately 116 million American adults.2 Seventy percent of all cancer patients experience significant pain during their illness.3 The financial impact of pain is staggering. In the United States, unrelieved pain and inadequate management of pain costs an estimated $560 billion to $635 billion each year in direct medical treatment costs and lost work productivity.2 Despite the high prevalence and costs of acute and chronic pain, inadequate pain management occurs. For example, approximately a third of patients enrolled in hospice reported pain at their last hospice visit.4 Cancer pain is often undertreated.5 Consequences of untreated pain include unnecessary suffering, physical and psychosocial dysfunction, immunosuppression, and sleep disturbances2 (Table 9-1). The varied reasons for the undertreatment of pain are discussed in this chapter. TABLE 9-1 HARMFUL EFFECTS OF UNRELIEVED ACUTE PAIN In 1968 Margo McCaffery, a nurse and pioneer in pain management, defined pain as “whatever the person experiencing the pain says it is, existing whenever the person says it does.”6 The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”2 With pain defined as a human experience, successful pain assessment and treatment must incorporate multiple dimensions.7 The biopsychosocial model of pain includes the physiologic, affective, cognitive, behavioral, and sociocultural dimensions of pain (Table 9-2). TABLE 9-2 DIMENSIONS OF PAIN The emotional distress of pain can cause suffering, which is the state of distress associated with loss. Suffering can result in a profound sense of insecurity and lack of control. When suffering occurs, people can experience spiritual distress. Achieving pain relief is an essential step in relieving suffering. In addition, the assessment of ways in which a person’s spirituality influences and is influenced by pain is important.8 Some people cope with pain by distracting themselves, whereas others convince themselves that the pain is permanent, untreatable, and overwhelming. People who believe that their pain is uncontrollable and overwhelming are more likely to have poor outcomes.9 Nociception is the physiologic process by which information about tissue damage is communicated to the central nervous system (CNS). It involves four processes: (1) transduction, (2) transmission, (3) perception, and (4) modulation (Fig. 9-1). In addition to stimulating nociceptors to fire, inflammation and the subsequent release of chemical mediators lower nociceptor thresholds. As a result, nociceptors may fire in response to stimuli that previously were insufficient to elicit a response. They may also fire in response to non-noxious stimuli, such as light touch. This increased susceptibility to nociceptor activation is called peripheral sensitization. Leukotrienes, prostaglandins, cytokines, and substance P are involved in peripheral sensitization. Cyclooxygenase (COX), an enzyme produced in the inflammatory response, also plays an important role in peripheral sensitization. A clinical example of this process is sunburn. This thermal injury causes inflammation that results in a sensation of pain when the affected skin is lightly touched. Peripheral sensitization also amplifies signal transmission, which in turn contributes to central sensitization (discussed under Dorsal Horn Processing). The pain produced from activation of peripheral nociceptors is called nociceptive pain (described later in the chapter on p. 119). Therapies that alter either the local environment or sensitivity of the peripheral nociceptors can prevent transduction and initiation of an action potential. Decreasing the effects of chemicals released at the periphery is the basis of several drug approaches to pain relief. For example, nonsteroidal antiinflammatory drugs (NSAIDs), such as ibuprofen (Advil, Motrin) and naproxen (Naprosyn, Aleve), and corticosteroids, such as dexamethasone (Decadron), exert their analgesic effects by blocking pain-sensitizing chemicals. NSAIDs block the action of COX, thereby interfering with the production of prostaglandins. Corticosteroids reduce the production of both prostaglandins and leukotrienes (see Fig. 12-2). The propagation of pain impulses from the site of transduction to the brain is shown in Fig. 9-1. Three segments are involved in nociceptive signal transmission: (1) transmission along the peripheral nerve fibers to the spinal cord, (2) dorsal horn processing, and (3) transmission to the thalamus and the cerebral cortex. The manner in which nerve fibers enter the spinal cord is central to the notion of spinal dermatomes. Dermatomes are areas on the skin that are innervated primarily by a single spinal cord segment. The distinctive pattern of the rash caused by herpes zoster (shingles) across the back and trunk is determined by dermatomes (see Fig. 24-7). Different dermatomes and their innervations are illustrated in eFig. 9-1 (available on the website for this chapter) and Fig. 56-6. Increased sensitivity and hyperexcitability of neurons in the CNS is called central sensitization. Peripheral tissue damage or nerve injury can cause central sensitization, and continued nociceptive input from the periphery is necessary to maintain it. As a result of the increased excitability of neurons within the CNS, normal sensory inputs cause abnormal sensing and responses to painful and other stimuli. This explains why some people experience significant pain from touch or tactile stimulation in and around the areas of tissue or nerve injury. This is called allodynia. With central sensitization, the central processing circuits are altered. In some cases, central sensitization can be long-lasting due to changes in the synapse.10 With ongoing stimulation of slowly conducting unmyelinated C-fiber nociceptors, firing of specialized dorsal horn neurons gradually increases. These inputs create many problems, including the sprouting of wide dynamic range (WDR) neurons and induction of glutamate-dependent N-methyl-d-aspartate (NMDA) receptors. WDR neurons respond to both nociceptive and non-nociceptive inputs that are of varying levels of stimulus intensity. When these neuron dendrites sprout, they grow into areas where pain-receiving nerve cell bodies are located. This results in the capacity to transmit a broader range of stimuli-producing signals, which are then passed up the spinal cord and brain. This process is known as windup and depends on the activation of NMDA receptors. NMDA receptor antagonists, such as ketamine (Ketalar), potentially interrupt or block mechanisms that lead to or sustain central sensitization. Windup, like central sensitization and hyperalgesia (increased pain responses to noxious stimuli), is induced by C-fiber inputs. Windup is different, however, in that it can be short lasting, whereas central sensitization and hyperalgesia persist over time.11 Neuroplasticity refers to processes that allow neurons in the brain to compensate for injury and adjust their responses to new situations or changes in their environment.12 Neuroplasticity contributes to adaptive mechanisms for reducing pain but also can result in maladaptive mechanisms that enhance pain. Genetic variability among individuals may have an important effect on the plasticity of the CNS.12 Understanding this phenomenon helps explain individual differences in response to pain and why some patients develop chronic pain conditions whereas others do not. Clinically, central sensitization of the dorsal horn results in (1) hyperalgesia, (2) painful responses to normally innocuous stimuli (allodynia), (3) prolonged pain after the original noxious stimulus ends (called persistent pain), and (4) the extension of tenderness or increased pain sensitivity outside of an area of injury to include uninjured tissue (i.e., expansion of nociceptive receptive fields, or secondary hyperalgesia).13 Referred pain must be considered when interpreting the location of pain reported by the person with an injury or a disease involving visceral organs. The location of a stimulus may be distant from the pain location reported by the patient (Fig. 9-2). For example, pain from liver disease is frequently located in the right upper abdominal quadrant, but can also be referred to the anterior and posterior neck region and to a posterior flank area. If referred pain is not considered when evaluating a pain location report, diagnostic tests and therapy could be misdirected. Modulation involves the activation of descending pathways that exert inhibitory or facilitatory effects on the transmission of pain (see Fig. 9-1). Depending on the type and degree of modulation, nociceptive stimuli may or may not be perceived as pain. Modulation of pain signals can occur at the level of the periphery, spinal cord, brainstem, and cerebral cortex. Descending modulatory fibers release chemicals such as serotonin, norepinephrine, GABA, and endogenous opioids that can inhibit pain transmission. Pain can be categorized in several ways. Most commonly, pain is categorized as nociceptive or neuropathic based on underlying pathology (Table 9-3). Another useful scheme is to classify pain as acute or chronic (Table 9-4). TABLE 9-3 COMPARISON OF NOCICEPTIVE AND NEUROPATHIC PAIN Adapted from National Institute of Neurological Disorders and Stroke: Complex regional pain syndrome fact sheet. Retrieved from www.ninds.nih.gov/disorders/reflex_sympathetic_dystrophy/detail_reflex_sympathetic_dystrophy.htm. CNS, Central nervous system; GI, gastrointestinal. *Note: Some types of neuropathic pain (e.g., postherpetic neuralgia) are caused by more than one neuropathologic mechanism. TABLE 9-4 DIFFERENCES BETWEEN ACUTE AND CHRONIC PAIN Adapted from National Institute of Neurological Disorders and Stroke: Complex regional pain syndrome fact sheet. Retrieved from www.ninds.nih.gov/disorders/reflex_sympathetic_dystrophy/detail_reflex_sympathetic_dystrophy.htm. Neuropathic pain is caused by damage to peripheral nerves or structures in the CNS.14 Typically described as numbing, hot, burning, shooting, stabbing, sharp, or electric shock–like, neuropathic pain can be sudden, intense, short lived, or lingering. Paroxysmal firing of injured nerves is responsible for shooting and electric shock–like sensations. Common causes of neuropathic pain include trauma, inflammation (e.g., secondary to a herniated disc inflaming the adjacent nerve and dorsal root ganglion), metabolic diseases (e.g., diabetes mellitus), alcoholism, infections of the nervous system (e.g., herpes zoster, human immunodeficiency virus), tumors, toxins, and neurologic diseases (e.g., multiple sclerosis). One particularly debilitating type of neuropathic pain is complex regional pain syndrome (CRPS). Typical features include dramatic changes in the color and temperature of the skin over the affected limb or body part, accompanied by intense burning pain, skin sensitivity, sweating, and swelling. CRPS type I is frequently triggered by tissue injury, surgery, or a vascular event such as stroke.14 CRPS type II includes all these features in addition to a peripheral nerve lesion. Neuropathic pain often is not well controlled by opioid analgesics alone. Treatment frequently necessitates a multimodal approach combining various adjuvant analgesics, including tricyclic antidepressants (e.g., nortriptyline [Pamelor], desipramine [Norpramin]), SNRIs (e.g., venlafaxine, duloxetine, bupropion [Wellbutrin, Zyban]), antiseizure drugs (e.g., gabapentin [Neurontin], pregabalin [Lyrica]), transdermal lidocaine, and α2-adrenergic agonists (e.g., clonidine [Catapres]). NMDA receptor antagonists such as ketamine have shown promise in alleviating neuropathic pain refractory to other drugs.14 Acute pain and chronic pain differ in their cause, course, manifestations, and treatment (see Table 9-4). Examples of acute pain include postoperative pain, labor pain, pain from trauma (e.g., lacerations, fractures, sprains), pain from infection (e.g., dysuria from cystitis), and pain from acute ischemia. For acute pain, treatment includes analgesics for symptom control and treatment of the underlying cause (e.g., splinting for a fracture, antibiotic therapy for an infection). Normally, acute pain diminishes over time as healing occurs. However, acute pain that persists can ultimately lead to disabling chronic pain states. For example, pain associated with herpes zoster (shingles) subsides as the acute infection resolves, usually within a month. However, sometimes the pain persists and develops into a chronic pain state called postherpetic neuralgia. Assessment is an essential, though often overlooked, step in pain management. Regularly screen all patients for pain and, when present, perform a more thorough pain assessment. The key to accurate and effective pain assessment is to consider the core principles of pain assessment (Table 9-5). TABLE 9-5 CORE PRINCIPLES OF PAIN ASSESSMENT Most components of a pain assessment involve direct interview or observation of the patient. Diagnostic studies and physical examination findings complete the initial assessment. Although the assessment differs according to the clinical setting, patient population, and point of care (i.e., whether the assessment is part of an initial workup or a reassessment of pain following therapy), the evaluation of pain should always be multidimensional (Table 9-6). TABLE 9-6 Determining the location of pain assists in identifying possible causes and treatment. Some patients may be able to specify the precise location(s) of their pain, whereas others may describe general areas or comment that they “hurt all over.” The location of the pain may also be referred from its origin to another site (see Fig. 9-2). For example, myocardial infarction can result in pain in the left shoulder. Pain may also radiate from its origin to another site. For example, angina pectoris can radiate from the chest to the jaw or down the left arm. This is referred to as radiating pain. Sciatica is pain that follows the course of the sciatic nerve. It may originate from joints or muscles around the back or from compression or damage to the sciatic nerve. The pain is projected along the course of the peripheral nerve, causing painful shooting sensations down the back of the thigh and inside of the leg to the foot. Assessing the severity, or intensity, of pain provides a reliable measure to determine the type of treatment and its effectiveness. Pain scales help the patient communicate pain intensity. Choice of a scale to use should be based on the patient’s developmental needs and cognitive status. Most adults can rate the intensity of their pain using numeric scales (e.g., 0 = no pain, 10 = the worst pain) or verbal descriptor scales (e.g., none, a little, moderate, severe). These tools are sometimes easier for patients to use if they are oriented vertically or include a visual component. The Pain Thermometer Scale is an example of this type of scale15 (Fig. 9-3). Other visual pain measures or scales include the Wong-Baker FACES Pain Rating Scale (see eFig. 9-3 on the website for this chapter) and the FACES Pain Scale–Revised (see eFig. 9-4 on the website for this chapter). These and other pain scales may be useful for patients with cognitive or language barriers to describe their pain.16 Pain assessment measures for cognitively impaired adults and nonverbal adults are addressed later in this chapter. Although intensity is an important factor in determining analgesic approaches, do not dose patients with opioids solely based on reported pain scores.17 Opioid “dosing by numbers” without taking into account a patient’s sedation level and respiratory status can lead to unsafe practices and serious adverse events. Safer analgesic administration can be achieved by balancing an amount of pain relief with analgesic side effects. Adjustments in therapy can be made to promote better pain control and minimize adverse outcomes. All pain treatment plans are based on the following 10 principles and practice standards: 1.Follow the principles of pain assessment (see Table 9-5). Remember that pain is a subjective experience. The patient is not only the best judge of his or her own pain, but also the expert on the effectiveness of each pain treatment. 2. Use a holistic approach to pain management. The experience of pain affects all aspects of a person’s life. Thus a holistic approach to assessment, treatment, and evaluation is required.18 3. Every patient deserves adequate pain management. Many patient populations, including ethnic minorities, older adults, and people with past or current substance abuse, are at risk for inadequate pain management. Be aware of your own biases and ensure that all patients are treated respectfully. 4. Base the treatment plan on the patient’s goals. Discuss with the patient realistic goals for pain relief during the initial pain assessment. Although goals can be described in terms of pain intensity (e.g., the desire for average pain to decrease from “8/10” to “3/10”), with chronic pain conditions functional goal setting should be encouraged (e.g., a goal of performing certain daily activities, such as socializing and hobbies). Over the course of prolonged therapy, reassess these goals and progress made toward meeting them. The patient, in collaboration with the health care team, determines new goals. If the patient has unrealistic goals for therapy, such as wanting to be completely rid of all chronic arthritis pain, work with the patient to establish a more realistic goal. 5. Use both drug and nondrug therapies. Although drugs are often considered the mainstay of therapy, incorporate self-care activities and nondrug therapies to increase the overall effectiveness of therapy and to allow for the reduction of drug dosages to minimize adverse drug effects.19 6. When appropriate, use a multimodal approach to analgesic therapy. Multimodal analgesia is the use of two or more classes of analgesic medications to take advantage of the various mechanisms of action. This approach achieves superior pain relief, enhances patient satisfaction, and decreases adverse effects of individual drugs.20 7. Address pain using an interdisciplinary approach. The expertise and perspectives of an interdisciplinary team are often necessary to provide effective evaluation and therapies for patients with pain, especially chronic pain. Interdisciplinary teams frequently include psychology, physical and occupational therapy, pharmacy, spiritual care, and multiple medical specialties (e.g., neurology, palliative care, oncology, surgery, anesthesiology). Some pain teams also include massage therapists, music therapists, acupuncturists, and art therapists. 8. Evaluate the effectiveness of all therapies to ensure that they are meeting the patient’s goals. Achievement of an effective treatment plan often requires trial and error. Adjustments in drug, dosage, or route are common to achieve maximal benefit while minimizing adverse effects. This trial-and-error process can become frustrating for the patient and caregivers. Reassure them that pain relief, if not pain cessation, is possible and that the health care team will continue to work with them to achieve adequate pain relief. 9. Prevent and/or manage medication side effects. Side effects are a major reason for treatment failure and nonadherence. Side effects are managed in one of several ways, as described in Table 9-7. You play a key role in monitoring for and treating side effects, and in teaching patients and caregivers how to minimize these effects. TABLE 9-7 DRUG THERAPY Managing Side Effects of Pain Medications
Pain
Magnitude of Pain Problem
Response
Possible Consequences
Endocrine and Metabolic
Cardiovascular
Respiratory
Renal and Urologic
Gastrointestinal
↓ Gastric and intestinal motility
Musculoskeletal
Neurologic
Impaired cognitive function
Immunologic
↓ Immune response
Infection
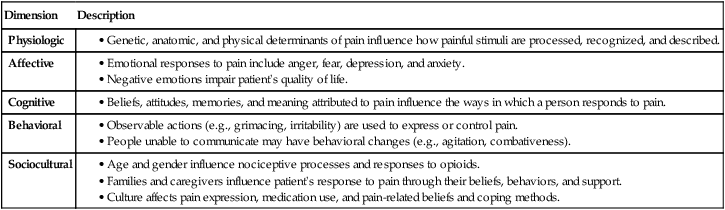
Definitions and Dimensions of Pain
Dimension
Description
Physiologic
Affective
Cognitive
Behavioral
Sociocultural
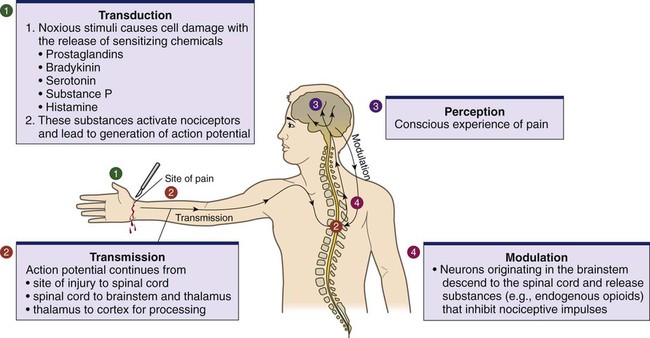
Pain Mechanisms
Transduction.
Transmission.
Transmission to Spinal Cord.
Dorsal Horn Processing.
Modulation.
Classification of Pain
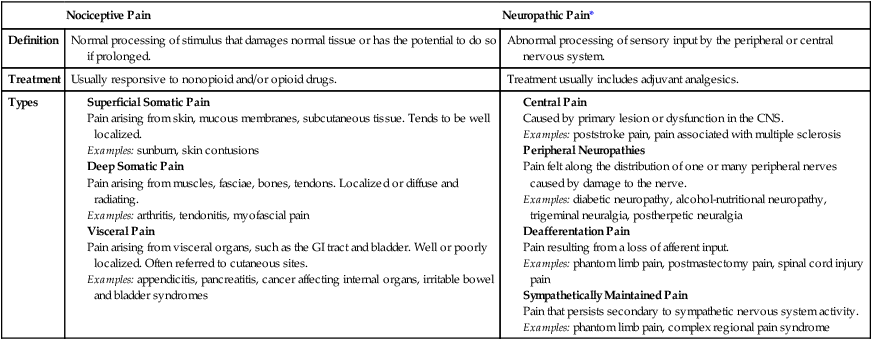
Acute Pain
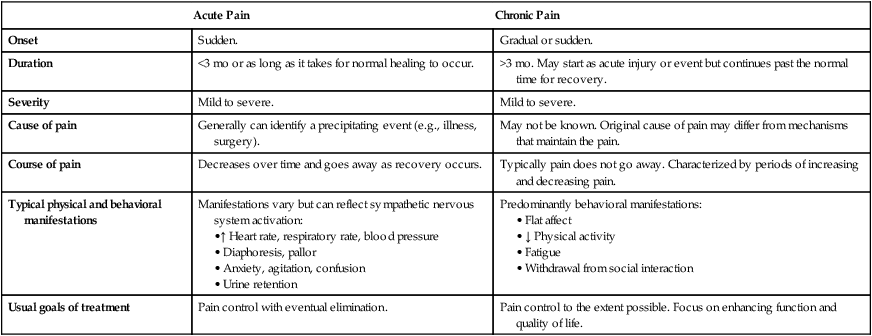
Chronic Pain
Onset
Sudden.
Gradual or sudden.
Duration
<3 mo or as long as it takes for normal healing to occur.
>3 mo. May start as acute injury or event but continues past the normal time for recovery.
Severity
Mild to severe.
Mild to severe.
Cause of pain
Generally can identify a precipitating event (e.g., illness, surgery).
May not be known. Original cause of pain may differ from mechanisms that maintain the pain.
Course of pain
Decreases over time and goes away as recovery occurs.
Typically pain does not go away. Characterized by periods of increasing and decreasing pain.
Typical physical and behavioral manifestations
Manifestations vary but can reflect sympathetic nervous system activation:
Predominantly behavioral manifestations:
Usual goals of treatment
Pain control with eventual elimination.
Pain control to the extent possible. Focus on enhancing function and quality of life.
Neuropathic Pain
Acute and Chronic Pain
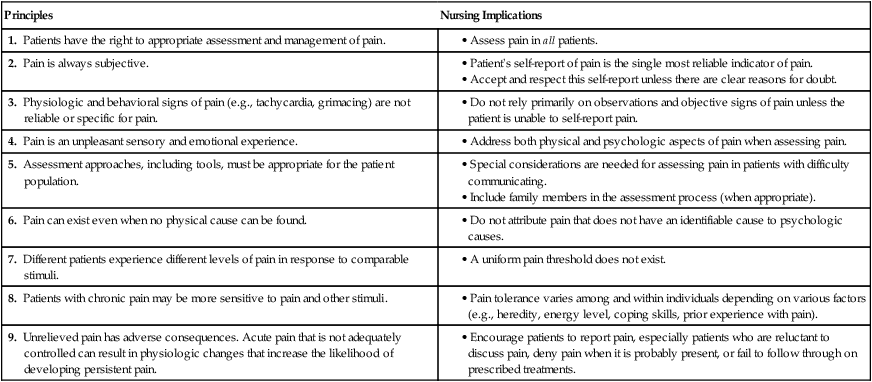
Pain Assessment
Principles
Nursing Implications
1. Patients have the right to appropriate assessment and management of pain.
2. Pain is always subjective.
3. Physiologic and behavioral signs of pain (e.g., tachycardia, grimacing) are not reliable or specific for pain.
4. Pain is an unpleasant sensory and emotional experience.
5. Assessment approaches, including tools, must be appropriate for the patient population.
6. Pain can exist even when no physical cause can be found.
7. Different patients experience different levels of pain in response to comparable stimuli.
8. Patients with chronic pain may be more sensitive to pain and other stimuli.
9. Unrelieved pain has adverse consequences. Acute pain that is not adequately controlled can result in physiologic changes that increase the likelihood of developing persistent pain.
Elements of a Pain Assessment
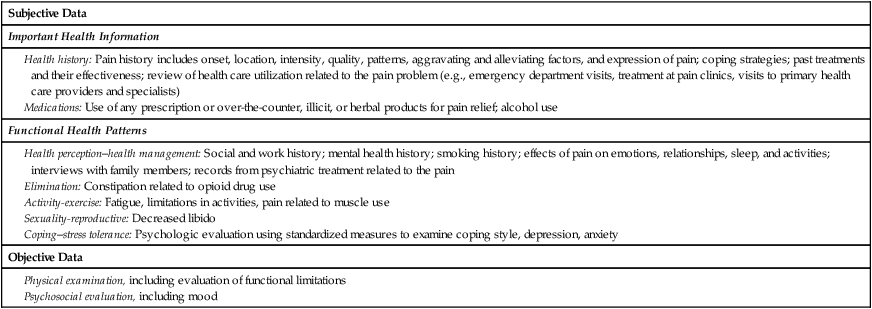
Location.
Intensity.
Pain Treatment
Basic Principles