CHAPTER 28 There are three main classes of opioid receptors, designated mu, kappa, and delta. From a pharmacologic perspective, mu receptors are the most important. Why? Because opioid analgesics act primarily by activating mu receptors, although they also produce weak activation of kappa receptors. As a rule, opioid analgesics do not interact with delta receptors. In contrast to opioid analgesics, endogenous opioid peptides act through all three opioid receptors, including delta receptors. Important responses to activation of mu and kappa receptors are summarized in Table 28–1. TABLE 28–1 Important Responses to Activation of Mu and Kappa Receptors Drugs that act at opioid receptors are classified on the basis of how they affect receptor function. At each type of receptor, a drug can act in one of three ways: as an agonist, partial agonist, or antagonist. (Recall from Chapter 5 that a partial agonist is a drug that produces low to moderate receptor activation when administered alone, but will block the actions of a full agonist if the two are given together.) Based on these actions, drugs that bind opioid receptors fall into three major groups: (1) pure opioid agonists, (2) agonist-antagonist opioids, and (3) pure opioid antagonists. The actions of drugs in these groups at mu and kappa receptors are summarized in Table 28–2. TABLE 28–2 Drug Actions at Mu and Kappa Receptors The pure opioid agonists activate mu receptors and kappa receptors. By doing so, the pure agonists can produce analgesia, euphoria, sedation, respiratory depression, physical dependence, constipation, and other effects. As indicated in Table 28–3, the pure agonists can be subdivided into two groups: strong opioid agonists and moderate to strong opioid agonists. Morphine is the prototype of the strong agonists. Codeine is the prototype of the moderate to strong agonists. TABLE 28–3 Opioid Analgesics: Abuse Liability and Maximal Pain Relief *CSA = Controlled Substances Act. †In the United States, hydrocodone is available only in combination with aspirin or acetaminophen. These combination products are classified under Schedule III. Four agonist-antagonist opioids are available: pentazocine, nalbuphine, butorphanol, and buprenorphine. The actions of these drugs at mu and kappa receptors are summarized in Table 28–2. When administered alone, the agonist-antagonist opioids produce analgesia. However, if given to a patient who is taking a pure opioid agonist, these drugs can antagonize analgesia caused by the pure agonist. Pentazocine [Talwin] is the prototype of the agonist-antagonists. The use of morphine and other opioids to relieve pain is discussed further in this Chapter (under Clinical Use of Opioids) and in Chapter 29 (Pain Management in Patients with Cancer). • Opioid peptides and morphine-like drugs both produce analgesia when administered to experimental subjects. • Opioid peptides and morphine-like drugs share structural similarities (Fig. 28–1). • Opioid peptides and morphine-like drugs bind to the same receptors in the CNS. • The receptors to which opioid peptides and morphine-like drugs bind are located in regions of the brain and spinal cord associated with perception of pain. • Subjects rendered tolerant to analgesia from morphine-like drugs show cross-tolerance to analgesia from opioid peptides. • The analgesic effects of opioid peptides and morphine-like drugs can both be blocked by the same antagonist: naloxone. Because of their effects on the intestine, opioids are highly effective for treating diarrhea. In fact, antidiarrheal use of these drugs preceded analgesic use by centuries. The impact of opioids on intestinal function is an interesting example of how an effect can be detrimental (constipation) or beneficial (relief of diarrhea) depending on who is taking the medication. Opioids employed specifically to treat diarrhea are discussed in Chapter 80. For individuals who are highly dependent, the abstinence syndrome can be extremely unpleasant. Initial reactions include yawning, rhinorrhea, and sweating. Onset occurs about 10 hours after the final dose. These early responses are followed by anorexia, irritability, tremor, and “gooseflesh”—hence the term cold turkey. At its peak, the syndrome manifests as violent sneezing, weakness, nausea, vomiting, diarrhea, abdominal cramps, bone and muscle pain, muscle spasm, and kicking movements—hence, “kicking the habit.” Giving an opioid at any time during withdrawal rapidly reverses all signs and symptoms. Left untreated, the morphine withdrawal syndrome runs its course in 7 to 10 days. It should be emphasized that, although withdrawal from opioids is unpleasant, the syndrome is rarely dangerous. In contrast, withdrawal from general CNS depressants (eg, barbiturates, alcohol) can be lethal (see Chapter 34). The abuse liability of the opioids is reflected in their classification under the Controlled Substances Act. (The provisions of this act are discussed in Chapter 37.) As shown in Table 28–3, morphine and all other strong opioid agonists are classified under Schedule II. This classification reflects a moderate to high abuse liability. The agonist-antagonist opioids have a lower abuse liability and hence are classified under Schedule IV (butorphanol, pentazocine) or Schedule V (buprenorphine), or have no classification at all (nalbuphine). Healthcare personnel who prescribe, dispense, and administer opioids must adhere to the procedures set forth in the Controlled Substances Act. The major interactions between morphine and other drugs are summarized in Table 28–4. Some interactions are adverse, and some are beneficial. TABLE 28–4 Interactions of Morphine-Like Drugs with Other Drugs In an effort to produce a strong analgesic with a low potential for respiratory depression and abuse, pharmaceutical scientists have created many new opioid analgesics. However, none of the newer pure opioid agonists can be considered truly superior to morphine: These drugs are essentially equal to morphine with respect to analgesic action, abuse liability, and the ability to cause respiratory depression. Also, to varying degrees, they all cause sedation, euphoria, constipation, urinary retention, cough suppression, hypotension, and miosis. However, despite their similarities to morphine, the newer drugs do have unique qualities. Hence one agent may be more desirable than another in a particular clinical setting. With all of the newer pure opioid agonists, toxicity can be reversed with an opioid antagonist (eg, naloxone). Important differences between morphine and the newer strong opioid analgesics are discussed below. Table 28–5 summarizes dosages, routes, and time courses for morphine and the newer agents. TABLE 28–5 Clinical Pharmacology of Pure Opioid Agonists
Opioid (narcotic) analgesics, opioid antagonists, and nonopioid centrally acting analgesics
Opioid analgesics
Introduction to the opioids
Terminology
Opioid receptors

Receptor Type
Response
Mu
Kappa
Analgesia
Respiratory depression
Sedation
Euphoria
Physical dependence
Decreased GI motility
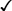
Classification of drugs that act at opioid receptors

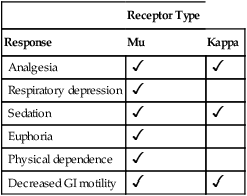
Receptor Type
Drugs
Mu
Kappa
Pure Opioid Agonists
Morphine, codeine, meperidine, and other morphine-like drugs
Agonist
Agonist
Agonist-Antagonist Opioids
Pentazocine, nalbuphine, butorphanol
Antagonist
Agonist
Buprenorphine
Partial agonist
Antagonist
Pure Opioid Antagonists
Naloxone, naltrexone, others
Antagonist
Antagonist
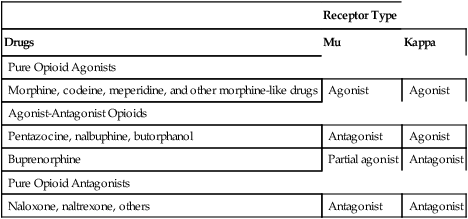
Pure opioid agonists.

Drug and Category
CSA* Schedule
Abuse Liability
Maximal Pain Relief
Strong Opioid Agonists
Alfentanil
II
High
High
Fentanyl
II
High
High
Hydromorphone
II
High
High
Levorphanol
II
High
High
Meperidine
II
High
High
Methadone
II
High
High
Morphine
II
High
High
Oxymorphone
II
High
High
Remifentanil
II
—
High
Sufentanil
II
High
High
Moderate to Strong Opioid Agonists
Codeine
II
Moderate
Low
Hydrocodone
III†
Moderate
Moderate
Oxycodone
II
Moderate
Moderate to high
Tapentadol
II
Moderate
Moderate to high
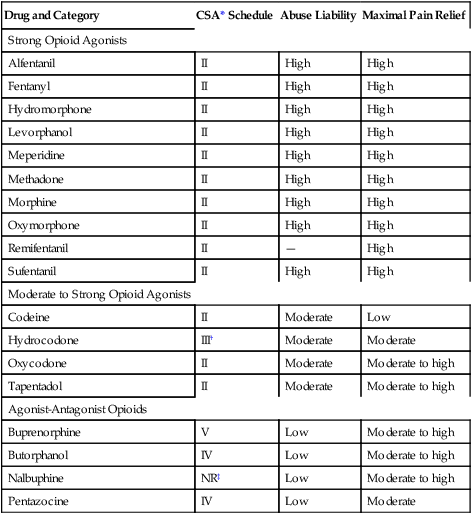
Agonist-Antagonist Opioids
Buprenorphine
V
Low
Moderate to high
Butorphanol
IV
Low
Moderate to high
Nalbuphine
NR‡
Low
Moderate to high
Pentazocine
IV
Low
Moderate
Agonist-antagonist opioids.
Basic pharmacology of the opioids
Morphine
Therapeutic use: relief of pain
Mechanism of analgesic action.
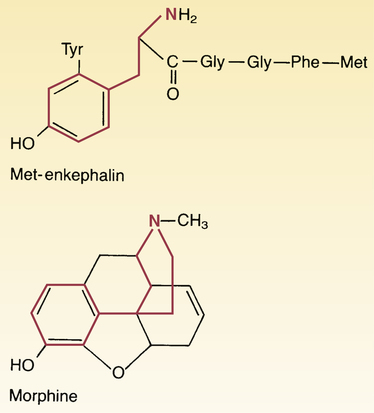
 Structural similarity between morphine and met-enkephalin.
Structural similarity between morphine and met-enkephalin.
In the morphine structural formula, highlighting indicates the part of the molecule thought responsible for interaction with opioid receptors. In the met-enkephalin structural formula, highlighting indicates the region of structural similarity with morphine.
Adverse effects
Constipation.
Tolerance and physical dependence
Physical dependence.
Abuse liability
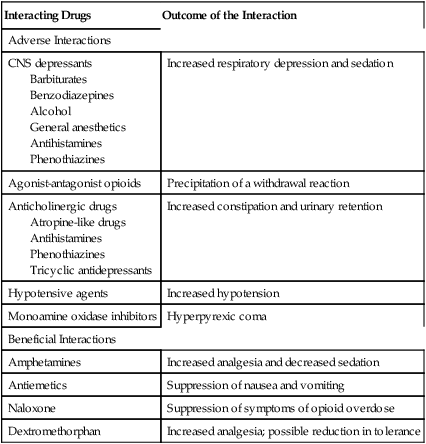
Drug interactions

Interacting Drugs
Outcome of the Interaction
Adverse Interactions
CNS depressants
Barbiturates
Benzodiazepines
Alcohol
General anesthetics
Antihistamines
Phenothiazines
Increased respiratory depression and sedation
Agonist-antagonist opioids
Precipitation of a withdrawal reaction
Anticholinergic drugs
Atropine-like drugs
Antihistamines
Phenothiazines
Tricyclic antidepressants
Increased constipation and urinary retention
Hypotensive agents
Increased hypotension
Monoamine oxidase inhibitors
Hyperpyrexic coma
Beneficial Interactions
Amphetamines
Increased analgesia and decreased sedation
Antiemetics
Suppression of nausea and vomiting
Naloxone
Suppression of symptoms of opioid overdose
Dextromethorphan
Increased analgesia; possible reduction in tolerance
Other strong opioid agonists

Time Course of Analgesic Effects
Drug and Route*
Equianalgesic Dose (mg)†
Onset (min)
Peak (min)
Duration (hr)
Codeine
PO
200
30–45
60–120
4–6
IM
130
10–30
30–60
4–6
SubQ
130
10–30
30–60
4–6
Fentanyl
IM
0.1
7–8
—
1–2
IV
0.1
—
—
0.5–1
Transdermal‡
—
Delayed
24–72
72
Transmucosal§
—
10–15
20
1–2
Nasal spray
—
10–15
15–20
1–2
Hydrocodone
PO
30
10–30
30–60
4–6
Hydromorphone
PO (IR)
7.5
30
90–120
4
PO (ER)
7.5
—
360–480
18–24
IM
1.5
15
30–60
4–5
IV
1.5
10–15
15–30
2–3
subQ
1.5
15
30–90
4
Levorphanol
PO
4
10–60
90–120
6–8
IM
2
—
60
6–8
IV
2
—
Within 20
6–8
subQ
2
—
60–90
6–8
Meperidine
PO
300
15
60–90
2–4
IM
75
10–15
30–50
2–4
IV
75
1
5–7
2–4
subQ
75
10–15
30–50
2–4
Methadone
PO
20
30–60
90–120
4–6¶
IM
10
10–20
60–120
4–5¶
IV
10
—
15–30
3–4¶
Morphine
PO (IR)
30
—
60–120
4–5
PO (ER)
30
—
420
8–12
IM
10
10–30
30–60
4–5
IV
10
—
20
4–5
subQ
10
10–30
50–90
4–5
Epidural
—
15–60
—
Up to 24
Intrathecal
—
15–60
—
Up to 24
Oxycodone
PO (IR)
20
15–30
60
3–4
PO (CR)
20
—
120–180
Up to 12
Oxymorphone
PO (IR)
10
—
—
4–6
PO (ER)
10
—
—
Up to 12
IM
1
10–15
30–90
3–6
IV
1
5–10
15–30
3–4
subQ
1
10–20
—
3–6
Rectal
10
15–30
120
3–6
Tapentadol
PO
100
45–60
90–120
4–8 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Opioid (narcotic) analgesics, opioid antagonists, and nonopioid centrally acting analgesics
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access
