(oh-bil-tox-AX-i-mab)
Anthim
Monoclonal antibody
pH 5.5
Usual dose
Premedication:
Administer diphenhydramine (Benadryl) before infusing obiltoxaximab. Although it does not prevent anaphylaxis, it may decrease the occurrence of other reported adverse reactions.
Obiltoxaximab:
A single dose of 16 mg/kg administered as an infusion over 90 minutes. See chart in Pediatric Dose for adults weighing less than 40 kg.
Pediatric dose
Premedication:
See Usual Dose.
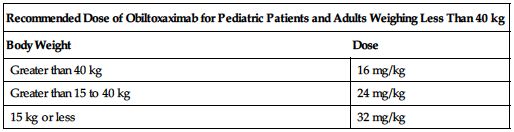
See the following chart for recommended weight-based dosing.
| Recommended Dose of Obiltoxaximab for Pediatric Patients and Adults Weighing Less Than 40 kg | |
| Body Weight | Dose |
| Greater than 40 kg | 16 mg/kg |
| Greater than 15 to 40 kg | 24 mg/kg |
| 15 kg or less | 32 mg/kg |
Dose adjustments
Age, gender, and race have no meaningful effect on the pharmacokinetics of obiltoxaximab.
Dilution
Available in single-dose vials containing 600 mg/6 mL (100 mg/mL). A clear to opalescent, colorless to pale yellow to pale brownish-yellow solution that may contain a few translucent-to white proteinaceous particulates. Do not shake. Sterile technique imperative; contains no preservatives. Obiltoxaximab solution must be diluted with NS in an appropriately sized infusion bag or syringe depending on the total volume required. Calculate the required dose:
Suggested dose × Patient weight (kg) = Required dose (mg)
Then calculate the required volume:
Calculated dose (mg) ÷ Concentration (100 mg/mL) = Required volume of obiltoxaximab (mL)
Infusion bag:
Withdraw a volume of solution from the infusion bag of NS equal to the calculated volume in mL of obiltoxaximab. Transfer the required volume (dose) of obiltoxaximab to the selected infusion bag. Gently invert the bag to mix the solution. Do not shake.
Syringe:
Using an appropriately sized syringe for the total volume of infusion to be administered, withdraw the calculated volume (dose) of obiltoxaximab into the syringe, then withdraw the appropriate amount of NS into the syringe. Gently mix the solution. Do not shake.
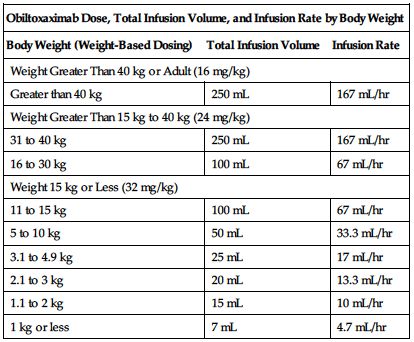
See the following chart for recommended dose, total infusion volume, and infusion rate by body weight.
| Obiltoxaximab Dose, Total Infusion Volume, and Infusion Rate by Body Weight | ||
| Body Weight (Weight-Based Dosing) | Total Infusion Volume | Infusion Rate |
| Weight Greater Than 40 kg or Adult (16 mg/kg) | ||
| Greater than 40 kg | 250 mL | 167 mL/hr |
| Weight Greater Than 15 kg to 40 kg (24 mg/kg) | ||
| 31 to 40 kg | 250 mL | 167 mL/hr |
| 16 to 30 kg | 100 mL | 67 mL/hr |
| Weight 15 kg or Less (32 mg/kg) | ||
| 11 to 15 kg | 100 mL | 67 mL/hr |
| 5 to 10 kg | 50 mL | 33.3 mL/hr |
| 3.1 to 4.9 kg | 25 mL | 17 mL/hr |
| 2.1 to 3 kg | 20 mL | 13.3 mL/hr |
| 1.1 to 2 kg | 15 mL | 10 mL/hr |
| 1 kg or less | 7 mL | 4.7 mL/hr |
Filters:
Use of a 0.22-micron in-line filter is required for administration.
Storage:
Before use, refrigerate at 2° to 8° C (36° to 46° F) in original carton to protect from light. Do not freeze. Do not shake. Infusion bag: Prepared solution stable for 4 hours at RT or refrigerated. Discard NS withdrawn from bag and discard unused portion in obiltoxaximab vials. Syringe: Administer immediately. Do not store solution in syringe. Discard unused product.
Compatibility
Specific information not available. Consider specific use; consult pharmacist.
Rate of administration
Administer through a 0.22-micron in-line filter.
A single dose as an infusion over 90 minutes. See chart in Dilution for recommended total infusion volumes and infusion rates for weight-based dosing. Flush the IV line with NS after the infusion.
Actions
A chimeric IgG1 kappa monoclonal antibody (mAb) that binds the protective antigen (PA) component of Bacillus anthracis toxin. It inhibits the binding of PA to its cellular receptors, thereby preventing the intracellular entry of the anthrax lethal factor and edema factor, the enzymatic toxin components responsible for the pathogenic effects of anthrax toxin. Steady-state volume of distribution is greater than plasma volume, suggesting some tissue distribution. Virtually no renal clearance occurs.
Indications and uses
Treatment of inhalational anthrax due to B. anthracis in adult and pediatric patients. Used in combination with appropriate antibacterial drugs (e.g., ciprofloxacin [Cipro], doxycycline, levofloxacin [Levaquin]). ■ Prophylaxis of inhalational anthrax due to B. anthracis when alternative therapies are not available or are not appropriate.
Limitation of use:
Should only be used for prophylaxis when its benefit for prevention of inhalational anthrax outweighs the risk of hypersensitivity and anaphylaxis. ■ Effectiveness is based solely on efficacy studies in animal models of inhalational anthrax. ■ No studies of the safety or pharmacokinetics (PK) have been done in the pediatric population. Dosing in pediatric patients was derived using a population pharmacokinetics approach designed to match the observed adult exposure to obiltoxaximab at a 16 mg/kg dose. ■ Does not have direct antibacterial activity. Should be used in combination with appropriate antibacterial drugs. ■ Not expected to cross the blood-brain barrier and does not prevent or treat meningitis.
Contraindications
Manufacturer states, “None.”
Precautions
For IV use only. ■ Administered by or under the direction of a physician knowledgeable in its use and in a facility equipped to monitor the patient and respond to any medical emergency. ■ Hypersensitivity reactions, including anaphylaxis, have been reported. ■ A therapeutic protein, there is a potential for immunogenicity.
Monitor:
Monitor for S/S of a hypersensitivity reaction (e.g., chest discomfort, cough, cyanosis, dyspnea, hypotension, postural dizziness, pruritus, rash, throat irritation, urticaria, wheezing) during the infusion and for some time following completion of the infusion. Reactions have occurred both during and after the infusion. ■ Premedication with diphenhydramine does not prevent anaphylaxis and may mask or delay the onset of symptoms of hypersensitivity.
Patient education:
Effectiveness has been studied only in animals with inhalational anthrax. ■ Immediately report any S/S of a hypersensitivity reaction (e.g., chest discomfort, cough, cyanosis, dyspnea, hypotension, postural dizziness, pruritus, rash, throat irritation, urticaria, wheezing) occurring during or after administration.
Maternal/child:
Pregnancy category B: use during pregnancy only if clearly needed. ■ Use caution during breast-feeding; effects unknown. ■ Safety and effectiveness for use in pediatric patients is based solely on efficacy studies in animal models; see Limitation of Use.
Elderly:
Numbers in clinical studies are insufficient to determine whether the elderly respond differently than younger subjects.
Drug/lab interactions
Coadministration of 16 mg/kg obiltoxaximab IV with IV or oral ciprofloxacin did not alter the pharmacokinetics of either ciprofloxacin or obiltoxaximab.
Side effects
The most frequently reported side effects in adults were cough, headache, infections of the upper respiratory tract, infusion site pain and/or swelling, nasal congestion, pain in extremity, pruritus, urticaria, and vessel puncture site bruising. Hypersensitivity reactions are the most serious side effect.
Antidote
Keep the physician informed of all side effects. Most will be treated symptomatically. If a hypersensitivity reaction occurs, discontinue the infusion immediately and treat with oxygen, epinephrine, antihistamines (e.g., IV diphenhydramine), corticosteroids, albuterol, vasopressors (e.g., dopamine), and ventilation equipment as indicated. No clinical experience with overdose. If overdose occurs, monitor patients for S/S of adverse effects. Resuscitate as necessary.
Obinutuzumab 
(oh-bi-nue-TOOZ-ue-mab)
Gazyva
Monoclonal antibodyAntineoplastic
pH 6
Usual dose
Hypotension may occur during obinutuzumab infusion. Consider withholding antihypertensive medications for 12 hours before and throughout each infusion and for the first hour after administration.
Premedication:
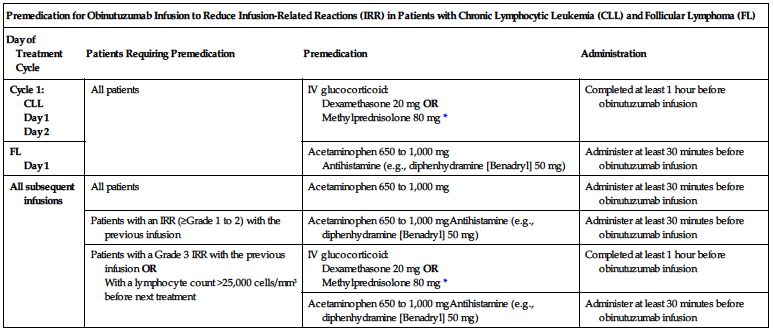
Premedication required. To prevent or attenuate severe hypersensitivity reactions, premedicate according to the following chart before the indicated infusion.
| Premedication for Obinutuzumab Infusion to Reduce Infusion-Related Reactions (IRR) in Patients with Chronic Lymphocytic Leukemia (CLL) and Follicular Lymphoma (FL) | |||
| Day of Treatment Cycle | Patients Requiring Premedication | Premedication | Administration |
| Cycle 1: CLL Day 1 Day 2 | All patients | IV glucocorticoid: Dexamethasone 20 mg OR Methylprednisolone 80 mg* | Completed at least 1 hour before obinutuzumab infusion |
| FL Day 1 | Acetaminophen 650 to 1,000 mg Antihistamine (e.g., diphenhydramine [Benadryl] 50 mg) | Administer at least 30 minutes before obinutuzumab infusion | |
| All subsequent infusions | All patients | Acetaminophen 650 to 1,000 mg | Administer at least 30 minutes before obinutuzumab infusion |
| Patients with an IRR (≥Grade 1 to 2) with the previous infusion | Acetaminophen 650 to 1,000 mgAntihistamine (e.g., diphenhydramine [Benadryl] 50 mg) | Administer at least 30 minutes before obinutuzumab infusion | |
| Patients with a Grade 3 IRR with the previous infusion OR With a lymphocyte count >25,000 cells/mm3 before next treatment | IV glucocorticoid: Dexamethasone 20 mg OR Methylprednisolone 80 mg* | Completed at least 1 hour before obinutuzumab infusion | |
| Acetaminophen 650 to 1,000 mgAntihistamine (e.g., diphenhydramine [Benadryl] 50 mg) | Administer at least 30 minutes before obinutuzumab infusion | ||
*Hydrocortisone is not recommended. It has not been effective in reducing the rate of infusion reactions.
Tumor lysis syndrome (TLS) prophylaxis:
Premedicate patients who have a high tumor burden, high circulating absolute lymphocyte counts (greater than 25,000 cells/mm3), or renal impairment with antihyperuricemics (e.g., allopurinol or rasburicase) before the start of therapy. Ensure adequate hydration. Continue prophylaxis before each subsequent obinutuzumab infusion as needed. ■ Antimicrobial prophylaxis is strongly recommended for patients with Grade 3 to 4 neutropenia lasting more than 1 week. Prophylaxis should continue until resolution of neutropenia to Grade 1 or 2. Consider antiviral and antifungal prophylaxis.
Obinutuzumab:
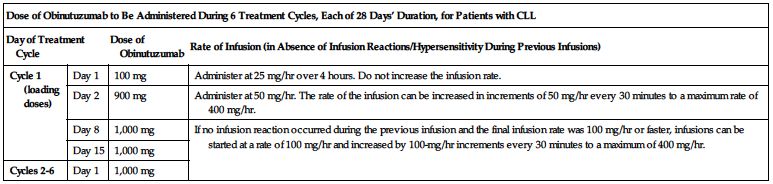
Doses must be administered as an infusion through a dedicated line according to the two charts on the next page.
| Dose of Obinutuzumab to Be Administered During 6 Treatment Cycles, Each of 28 Days’ Duration, for Patients with CLL | |||
| Day of Treatment Cycle | Dose of Obinutuzumab | Rate of Infusion (in Absence of Infusion Reactions/Hypersensitivity During Previous Infusions) | |
| Cycle 1 (loading doses) | Day 1 | 100 mg | Administer at 25 mg/hr over 4 hours. Do not increase the infusion rate. |
| Day 2 | 900 mg | Administer at 50 mg/hr. The rate of the infusion can be increased in increments of 50 mg/hr every 30 minutes to a maximum rate of 400 mg/hr. | |
| Day 8 | 1,000 mg | If no infusion reaction occurred during the previous infusion and the final infusion rate was 100 mg/hr or faster, infusions can be started at a rate of 100 mg/hr and increased by 100-mg/hr increments every 30 minutes to a maximum of 400 mg/hr. | |
| Day 15 | 1,000 mg | ||
| Cycles 2-6 | Day 1 | 1,000 mg | |
| Dose of Obinutuzumab to Be Administered During 6 Treatment Cycles, Each of 28 Days’ Duration, Followed by Obinutuzumab Monotherapy for Patients with FL | |||
| Day of Treatment Cycle | Dose of Obinutuzumab | Rate of Infusion (in Absence of Infusion Reactions/Hypersensitivity During Previous Infusions) | |
| Cycle 1 (loading doses) | Day 1 | 1,000 mg | Administer at 50 mg/hr. The rate of the infusion can be increased in increments of 50 mg/hr every 30 minutes to a maximum rate of 400 mg/hr. |
| Day 8 | 1,000 mg | If no infusion reaction occurred during the previous infusion and the final infusion rate was 100 mg/hr or faster, infusions can be started at a rate of 100 mg/hr and increased by 100-mg/hr increments every 30 minutes to a maximum of 400 mg/hr. | |
| Day 15 | 1,000 mg | ||
| Cycles 2 to 6 | Day 1 | 1,000 mg | |
| Monotherapy* | Every 2 months for 2 years | 1,000 mg | |
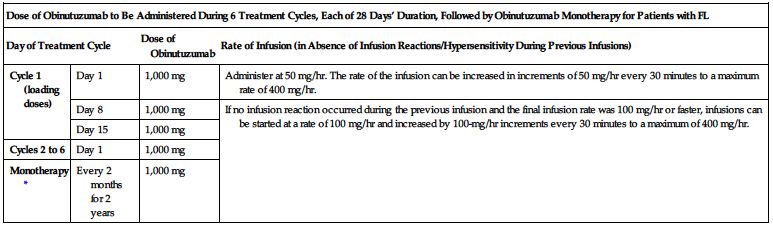
*Patients with FL who achieve stable disease, complete response, or partial response to the initial 6 cycles of obinutuzumab in combination with bendamustine should continue obinutuzumab as monotherapy for 2 years.
Dose adjustments
Consider treatment interruption if patients experience an infection, Grade 3 or 4 cytopenia, or a Grade 2 or greater nonhematologic toxicity. ■ In patients with Grade 3 or 4 thrombocytopenia, consider a dose delay of obinutuzumab and chemotherapy or a dose reduction of chemotherapy. ■ If a planned dose is missed during the first 6 cycles, administer as soon as possible and adjust dosing schedule accordingly. If appropriate, patients being treated for CLL who do not complete the Day 1 Cycle 1 dose may proceed to the Day 2 Cycle 1 dose. If a dose is missed during monotherapy in a patient being treated for FL, maintain the original dosing schedule for subsequent doses. ■ No dose adjustment indicated based on age, weight, or moderate renal impairment. Has not been studied in patients with a CrCl less than 30 mL/min or in patients with hepatic impairment.
Dilution
Available in a 1,000 mg/40 mL (25 mg/mL) single-use vial. Aseptic technique imperative.
CLL:
Preparation of solution for infusion on day 1 (100 mg) and day 2 (900 mg) of cycle 1:
Withdraw 4 mL (100 mg) of obinutuzumab solution from the vial. Inject into a 100-mL infusion bag of NS for immediate administration. Withdraw the remaining 36 mL (900 mg) of solution from the vial and inject into a 250-mL infusion bag of NS for use on Day 2. Mix infusion bags by gentle inversion. Do not shake. Clearly label each infusion bag; see Storage.
CLL and FL:
Preparation of 1,000-mg dose:
Withdraw 40 mL of obinutuzumab solution from the vial. Inject into a 250-mL infusion bag of NS. Mix by gentle inversion. Do not shake or freeze. Should be used immediately; see Storage. Final concentrations of 0.4 to 4 mg/mL are suitable for administration.
Filters:
Specific information not available.
Storage:
Refrigerate single-use vials at 2° to 8° C (36° to 46° F) in carton to protect from light. Do not freeze. Do not shake. If not used immediately, infusions diluted for use may be refrigerated for up to 24 hours, followed by 48 hours (including infusion time) at RT. Allow to come to room temperature, then use immediately.
Compatibility
Manufacturer states, “Dilute into a 0.9% sodium chloride (NS) PVC or non-PVC polyolefin infusion bag. Do not use other diluents such as dextrose (5%),” “Do not mix with other drugs,” “ Administer through a dedicated line,” and “No incompatibilities with polyvinylchloride (PVC) or non-PVC polyolefin bags and administration sets have been observed.”
Rate of administration
Do not administer as an IV push or bolus. See Usual Dose for specific rates of infusion. If an infusion reaction occurs, adjust the rate as follows.
Grade 1-2 (mild to moderate):
Reduce or interrupt infusion and treat as indicated. With resolution of symptoms, continue or resume infusion. If no further reactions occur, rate may escalate as indicated for each treatment-cycle dose.
Grade 3 (severe):
Interrupt infusion and manage symptoms. With resolution of symptoms, consider restarting the infusion at no more than half the rate that caused the reaction. If no further reactions occur, rate may escalate as indicated for each treatment-cycle dose. Discontinue permanently if a reaction occurs on rechallenge.
Grade 4 (life-threatening):
Stop infusion immediately and discontinue therapy permanently.
Actions
An antineoplastic agent. A humanized anti-CD20 monoclonal antibody of the IgG1 subclass produced by recombinant DNA technology. It targets the CD20 antigen expressed on the surface of pre B-lymphocytes and mature B-lymphocytes. Upon binding to CD20, it mediates B-cell lysis through several processes, resulting in cell death. Causes CD19 B-cell depletion. Recovery of CD19 B-cells may occur approximately 9 months after the last obinutuzumab dose, but some patients remain depleted at 18 months. Elimination occurs by a linear clearance pathway and a time-dependent nonlinear clearance pathway. Terminal half-life ranges from 26.4 to 36.8 days.
Indications and uses
Treatment of patients with previously untreated chronic lymphocytic leukemia (CLL) in combination with chlorambucil (Leukeran). ■ Treatment of patients with follicular lymphoma (FL) who relapsed after or are refractory to a rituximab-containing regimen. Given in combination with bendamustine followed by obinutuzumab monotherapy.
Contraindications
Manufacturer states, “None.”
Precautions
For IV infusion only; do not administer as an IV push or bolus. ■ Should be administered by or under the direction of the physician specialist in facilities equipped to monitor the patient and respond to any medical emergency. ■ Hepatitis B virus reactivation with fulminant hepatitis, hepatic failure, and death can occur in patients treated with anti-CD20–directed cytolytic antibodies, including obinutuzumab. For patients who show evidence of hepatitis B infection, consult physician with expertise in managing hepatitis B regarding monitoring and consideration for HBV antiviral therapy; see Monitor. ■ Progressive multifocal leukoencephalopathy (PML), including fatal PML, can occur in patients receiving obinutuzumab. JC virus infection resulting in PML was observed in patients treated with obinutuzumab. ■ Severe and life-threatening infusion reactions have occurred within 24 hours of the first 1,000 mg infused and with subsequent infusions. ■ Tumor lysis syndrome (TLS) has been reported within 12 to 24 hours after the first obinutuzumab infusion. S/S may include rapid reduction in tumor volume, renal insufficiency, hyperkalemia, hypocalcemia, hyperuricemia, or hyperphosphatemia. May cause acute renal failure requiring dialysis and has been fatal. ■ TLS occurs more often in patients with a high tumor burden, a high circulating lymphocyte count (greater than 25,000/mm3), or renal impairment. Consider prophylactic measures; see Monitor and Usual Dose. ■ Serious bacterial, fungal, and new or reactivated viral infections can occur during and/or following therapy. Do not administer to patients with an active infection. Patients with a history of recurring or chronic infections may be at increased risk for infection. ■ Severe and life-threatening neutropenia has been reported during treatment with obinutuzumab. May be delayed (occurring more than 28 days after completion of treatment and/or prolonged (lasting longer than 28 days). Anticipate, evaluate, and treat any S/S of developing infection. Consider administration of granulocyte colony-stimulating factors (G-CSF) in patients with Grade 3 or 4 neutropenia. ■ Severe and life-threatening thrombocytopenia has been reported during treatment with obinutuzumab in combination with chlorambucil or bendamustine. Fatal hemorrhagic events have occurred during Cycle 1 in patients with CLL being treated with obinutuzumab. ■ Has not been studied in patients with a CrCl less than 30 mL/min or in patients with hepatic impairment. ■ See Drug/Lab Interactions.
Monitor:
Obtain baseline CBC and platelet count and monitor at regular intervals. Repeat more frequently in patients who develop cytopenias (e.g., leukopenia, neutropenia, thrombocytopenia). ■ Screen all patients for HBV infection before treatment initiation. Monitor HBV-positive patients during and after treatment with obinutuzumab. Monitor patients with evidence of current or prior HBV infection for clinical and laboratory signs of hepatitis or HBV reactivation during and for several months after treatment with obinutuzumab. ■ Insufficient data exist regarding the safety of resuming obinutuzumab therapy in patients who develop HBV reactivation; see Antidote. ■ Consider PML in patients with new-onset or changes to pre-existing neurologic manifestations; consultation with a neurologist, brain MRI, and lumbar puncture may be required for diagnosis. ■ Monitor closely for S/S of an infusion reaction (e.g., bronchospasm, chills, diarrhea, dizziness, dyspnea, fatigue, fever, flushing, headache, hypertension, hypotension, laryngeal edema, larynx and throat irritation, nausea, tachycardia, vomiting, and wheezing). See Antidote for responses to various grades of an infusion reaction. ■ Patients with pre-existing cardiac or pulmonary conditions may be at greater risk for severe infusion reactions; monitor closely during and after the infusion. ■ Monitor patients for TLS, especially during the initial days of obinutuzumab treatment. Prevention and treatment of hyperuricemia due to TLS may be accomplished with adequate hydration and, if necessary, allopurinol or rasburicase and alkalinization of urine. Monitor electrolytes, renal function, and fluid status. Correct electrolyte abnormalities and provide supportive care, including dialysis, as indicated. ■ Observe closely for signs of infection. Patients with severe and long-lasting (greater than 1 week) neutropenia are strongly recommended to receive antimicrobial prophylaxis until resolution of neutropenia to Grade 1 or 2. Antiviral and antifungal prophylaxis should also be considered. ■ Monitor for thrombocytopenia (platelet count less than 50,000/mm3) and hemorrhagic events, especially during the first cycle. Initiate precautions to prevent excessive bleeding (e.g., inspect IV sites, skin, and mucous membranes; use extreme care during invasive procedures; test urine, emesis, stool, and secretions for occult blood).
Patient education:
Avoid pregnancy; see Maternal/Child. ■ Discuss health history (e.g., presence of an infection, carrier of or previous infection with hepatitis B virus, recent or scheduled vaccinations) and prescription and nonprescription medications with the health care provider administering the obinutuzumab. ■ Avoid vaccinations with live virus vaccines. ■ Review monitoring requirements and potential side effects before therapy. ■ Promptly report S/S of infection (e.g., fever, cough). ■ Promptly report S/S of infusion reactions, including breathing problems, chest pain, chills, diarrhea, dizziness, fever, nausea, and vomiting. ■ Promptly report S/S of TLS, including diarrhea, lethargy, nausea, and vomiting, and S/S of hepatitis, including worsening fatigue or yellow discoloration of skin or eyes. ■ Promptly report new neurologic S/S (e.g., changes in vision, loss of balance or coordination, disorientation, or confusion); could be warning signs of PML. ■ Promptly report S/S of hepatitis (e.g., fatigue, jaundice). ■ See Appendix D, p. 1333.
Maternal/child:
Use during pregnancy only if the potential benefit justifies the potential risk to the fetus. Obinutuzumab is likely to cause fetal B-cell depletion. Safety and timing of administration of live virus vaccines to infants born to mothers who were administered obinutuzumab during pregnancy require consultation with health care professionals. ■ Weigh risk versus benefit when considering breast-feeding. ■ Safety and effectiveness for use in pediatric patients not established.
Elderly:
In clinical trials, the incidence of serious adverse events was higher in older patients. Effectiveness was similar to that seen in younger adults.
Drug/lab interactions
Formal drug interaction studies have not been performed. ■ Avoid vaccinations with live or attenuated virus vaccines. Immunization with live virus vaccines is not recommended during treatment and until B-cell recovery. ■ Consider withholding concomitant medications that may increase the risk of bleeding (e.g., platelet inhibitors, anticoagulants), especially during the first cycle. ■ Hypotension may occur during obinutuzumab infusion. Consider withholding antihypertensive medications for 12 hours before and throughout each infusion and for the first hour after administration.
Side effects
Anemia, cough, diarrhea, fever, infusion reactions, nausea, neutropenia, and thrombocytopenia are the most commonly reported adverse reactions in patients with CLL or FL. Arthralgia, asthenia, constipation, decreased appetite, fatigue, sinusitis, upper respiratory tract infections, urinary tract infections, and vomiting were also commonly reported in patients with FL. Infusion reactions may be life-threatening. Hepatitis B reactivation, PML, tumor lysis syndrome, and serious infections have all been reported and may be life threatening. Back pain; dyspepsia; fatal hemorrhagic events (first cycle); hyperkalemia; hypoalbuminemia; hypocalcemia; hypokalemia; hyponatremia; hypophosphatemia; increased ALT, AST, alkaline phosphatase, and creatinine; leukopenia; lymphopenia; nasal congestion; nasopharyngitis; pain in extremities; pruritus; and worsening of pre-existing cardiac conditions have also been reported.
Antidote
Keep physician informed of all side effects. May constitute a medical emergency or will be treated symptomatically as indicated. Interrupt or reduce rate of infusion for Grade 1 or 2 infusion reactions and manage symptoms. Interrupt therapy for Grade 3 infusion reactions until resolution of symptoms. Permanently discontinue therapy for any Grade 4 infusion reaction including, but not limited to, anaphylaxis. Severe infusion reactions may require epinephrine (Adrenalin), antihistamines (e.g., diphenhydramine [Benadryl]), corticosteroids (e.g., dexamethasone [Decadron]), oxygen or bronchodilators (e.g., albuterol). Maintain a patent airway. In patients who have a Grade 1, 2, or 3 infusion reaction, see Rate of Administration for rate adjustments required if treatment is to be continued. In patients who develop reactivation of HBV, immediately discontinue obinutuzumab and concomitant chemotherapy and institute appropriate treatment. Resumption of therapy should be discussed with physicians with expertise in managing hepatitis B. Discontinue obinutuzumab and consider discontinuation or reduction of concomitant chemotherapy or immunosuppressive therapy in patients who develop PML. Consider treatment interruption in the event of infection, Grade 3 or 4 cytopenia, or a hematologic toxicity equal to or greater than Grade 2. Dose delays of obinutuzumab and chemotherapy or dose reductions of chemotherapy may be indicated. Consider administration of granulocyte colony-stimulating factors (G-CSF) in patients with Grade 3 or 4 neutropenia. Tumor lysis syndrome requires correction of electrolyte abnormalities and monitoring of renal function and fluid balance. Supportive care, including dialysis, may be required. Thrombocytopenia may require transfusion of blood products (i.e., platelet transfusion). Resuscitate as indicated.
Octreotide acetate
(ok-TREE-oh-tide AS-ah-tayt)
Octreotide Acetate PF, Sandostatin
Antidiarrheal
Growth hormone suppressant
pH 3.9 to 4.5
Usual dose
Usually given SC. Check label and confirm for IV use; Sandostatin LAR Depot is for IM use only; see Precautions. In most situations begin with a lower dose to allow gradual tolerance to GI side effects. Increase gradually based on patient response and tolerance. Begin SC or IM (LAR Depot) dosing as soon as practical.
Antidiarrheal (GI tumor):
50 mcg once or twice daily. Increase gradually if indicated.
Antidiarrheal (unlabeled for AIDS):
100 mcg as an IV bolus over 10 minutes. Follow with a continuous infusion, intermittent infusion, or bolus dose of 10 mcg/hr. Increase gradually to 100 mcg/hr. When adequate control is achieved, decrease to 75 mcg/hr. SC dose range is from 100 mcg up to 3,000 mcg/24 hr.
Carcinoid tumors:
100 to 600 mcg/24 hr in equally divided doses 2 to 4 times daily during first 2 weeks of therapy (50 to 300 mcg every 12 hours or 25 to 150 mcg every 6 hours). Average total daily dose ranges from 300 to 450 mcg, but therapeutic response is obtained with ranges from 50 to 750 mcg. Up to 1,500 mcg/day has been used in selected patients.
Carcinoid crisis (unlabeled):
100 mcg as an IV bolus. May be given to treat carcinoid crisis during anesthesia or given before induction of anesthesia as a prophylactic measure.
Vasoactive intestinal peptide tumors:
Average dose range is 200 to 300 mcg/24 hr in equally divided doses 2 to 4 times daily during first 2 weeks of therapy (100 to 150 mcg every 12 hours or 50 to 75 mcg every 6 hours). Average total daily dose ranges from 150 to 750 mcg but therapeutic response usually achieved with doses under 450 mcg/24 hr.
Treatment of GI bleeding (unlabeled):
Begin with a loading dose of 50 to 100 mcg. Follow with a continuous infusion of 25 to 50 mcg/hr for 1 to 5 days. Intermittent infusion or bolus doses may be substituted for the continuous infusion.
Growth hormone suppression (acromegaly):
50 mcg every 8 hours is the initial dose. Increase dose gradually as indicated by IGF-1 levels; see Monitor. Acromegaly has been suppressed at doses of 300 to 500 mcg/24 hr. May be maintained at home with infusions through an implantable IV or SC pump or SC dosing of 50 to 100 mcg every 8 hours.
Antihypoglycemic: Life-threatening hypoglycemia secondary to insulinoma (unlabeled):
100 mcg as an IV bolus.
Reduce output from GI or pancreatic fistulas (unlabeled):
50 to 200 mcg every 8 hours. In one study, 250 mcg/hr was given as a continuous infusion for 48 hours, followed with SC dosing. Fistula became dry within 72 hours and eventually closed.
Pediatric dose
Experience is limited, and doses are unlabeled. See Maternal/Child.
Intractable diarrhea:
1 to 10 mcg/kg of body weight/24 hr. May be given as a single daily dose or divided and given every 12 hours. Dose may be increased within the recommended range by 0.3 mcg/kg/dose every 3 days as needed. Maximum dose is 1,500 mcg/24 hr.
Diarrhea associated with graft versus host:
1 mcg/kg/dose bolus followed by 1 mcg/kg/hr as a continuous infusion has been used.
Dose adjustments
In all situations dose adjustment may be required on a daily basis to maintain symptomatic control. After initial 2 weeks of therapy, gradually decrease dose to achieve therapeutically effective maintenance dose. ■ Reduce dose in the elderly; half-life extended and clearance decreased. Start at the lower end of the dosing range. Consider the greater frequency of decreased organ function and of concomitant disease or drug therapy. ■ Half-life markedly extended in severe renal failure requiring dialysis. Reduction of maintenance dose indicated. ■ See Drug/Lab Interactions.
Dilution
Available in several different concentrations and formulations; read label carefully. May be given undiluted or may be diluted with 50 to 200 mL of NS or D5W and given as an intermittent infusion or further diluted and given as a continuous infusion.
Storage:
Before use store in refrigerator (2° to 8° C [36° to 46° F]) or at CRT for 14 days; protect from light. May store at room temperature on day of use. Diluted solution stable for 24 hours. Multidose vial must be dated on opening and discarded after 14 days.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer lists TPN as incompatible (forms a conjugate that decreases effectiveness). If used as an additive with insulin, octreotide markedly increases adsorption of insulin and reduces insulin availability.
One source suggests the following compatibilities:
Additive:
Heparin.
Y-site:
6% Hydroxyethyl starch (Voluven), pantoprazole (Protonix IV).
Rate of administration
IV injection:
A single dose over 3 minutes.
Intermittent infusion:
A single dose over 15 to 30 minutes.
Continuous infusion:
Give at a rate consistent with the required hourly dose in an amount of fluid appropriate for the specific patient.
Actions
A long-acting octapeptide. Mimics the actions of the natural hormone somatostatin, suppressing secretion of serotonin, gastroenteropancreatic peptides (e.g., gastrin, vasoactive intestinal peptide, insulin, glucagon, secretin, motilin, pancreatic polypeptide), and growth hormone. Decreases splanchnic blood flow. Stimulates fluid and electrolyte absorption from GI tract and prolongs GI transit time. These pharmacologic actions provide a means of treating the symptoms associated with metastatic carcinoid tumors (flushing and diarrhea) and vasoactive intestinal peptide (VIP)–secreting tumors (watery diarrhea). Other actions include inhibition of gallbladder contractility and bile secretion and suppression of thyroid-stimulating hormone (TSH) secretion. Distribution from plasma is rapid. About 65% bound to plasma protein. Half-life longer than the natural hormone (1.7 to 1.9 hours compared to 1 to 3 minutes). Action may extend to 12 hours. Some excreted unchanged in urine.
Indications and uses
To suppress or inhibit the severe diarrhea and flushing episodes associated with carcinoid tumor. ■ Treatment of profuse watery diarrhea associated with vasoactive intestinal peptide tumors (VIPomas). ■ Treatment of acromegaly to suppress growth hormone and achieve normalization of growth hormone and IGF-1 levels.
Unlabeled uses:
Treatment of severe diarrhea in patients with AIDS; treatment of chemotherapy-induced diarrhea. Carcinoid crisis during anesthesia, adjunct to treatment of life-threatening hypoglycemia, treatment of GI bleeding. Adjunct to pancreatectomy and treatment of GI or pancreatic fistulas.
Contraindications
Sensitivity to octreotide acetate or any of its components.
Precautions
IV use is limited to emergency situations. SC injection with rotation of injection sites is preferred route of administration. ■ Sandostatin LAR Depot must be administered intragluteally but has the advantage of extending the interval between injections to every 4 weeks. ■ May decrease size of tumors and slow rate of growth and metastases. Data not definitive. ■ May inhibit gallbladder contractility and decrease bile secretion. ■ Use caution in patients with diabetes. In patients with Type 1 diabetes, octreotide is likely to affect glucose regulation, and insulin requirements may be decreased. Severe, symptomatic hypoglycemia has been reported. In nondiabetics and Type 2 diabetics with partially intact insulin reserves, octreotide may result in decreased insulin levels and hyperglycemia. Glucose tolerance and antidiabetic treatment should be closely monitored. ■ Cardiac abnormalities (e.g., arrhythmias, bradycardia, conduction abnormalities, QT prolongation) have been reported. More common in patients with acromegaly; see Side Effects. ■ Suppresses thyroid-stimulating hormone. May cause hypothyroidism.
Monitor:
Observe for transient hyperglycemia or hypoglycemia during induction and dose changes because of changes in balance of hormones (e.g., insulin, glucagon, and growth hormone). ■ Monitor fluids and electrolytes carefully. ■ 5-HIAA, plasma serotonin, and plasma substance P may be useful lab studies to evaluate patient response with carcinoid tumor. Measurement of plasma vasoactive intestinal peptide will be helpful in VIPoma. ■ In acromegaly, initial response may be monitored with growth hormone levels at 1- to 4-hour intervals for 8 to 12 hours after a dose. IGF-1 levels every 2 weeks and/or multiple growth hormone levels taken 0 to 8 hours after administration may be used to make dose adjustments. Goal is to achieve growth hormone levels less than 5 ng/mL or IGF-1 (somatomedin C) levels less than 1.9 units/mL in males and less than 2.2 units/mL in females. After stabilization, IGF-1 or growth hormone levels should be re-evaluated at 6-month intervals. ■ In patients with acromegaly who have received irradiation, octreotide should be withdrawn yearly for 4 weeks to assess disease activity. If growth hormone or IGF-1 levels increase and S/S recur, octreotide therapy should be resumed. ■ Can alter fat absorption and decrease gallbladder motility; observe for gallbladder disease. Baseline and periodic ultrasound of gallbladder and bile ducts indicated in long-term SC therapy. Periodic fecal fat and carotene studies also indicated. ■ Pancreatitis has been reported. Monitor pancreatic enzymes as indicated. ■ Depressed B12 levels have been observed. Monitor in prolonged therapy. ■ Monitor baseline and periodic thyroid function tests (TSH, total, and/or free T4), especially in long-term SC therapy. ■ See Dose Adjustments and Drug/Lab Interactions.
Patient education:
Instruct patient and/or family in appropriate skills if self-administration indicated. To avoid or lessen incidence of GI side effects, schedule injections between meals and at bedtime. ■ In women with acromegaly being treated with octreotide, normalization of GH and IGF-1 may restore fertility. Adequate contraception is recommended.
Maternal/child:
Category B: although studies do not indicate harm to the fetus or infants, use in pregnancy and breast-feeding only if clearly needed. ■ Safety and effectiveness for use in pediatric patients not established; see Literature. In post-marketing reports, hypoxia, necrotizing enterocolitis, and death have been reported, most notably in pediatric patients under 2 years of age, many of which had serious underlying comorbid conditions. Relationship to octreotide not established.
Elderly:
See Dose Adjustments. ■ Response similar to that seen in younger patients; however, may be more sensitive to side effects; observe carefully.
Drug/lab interactions
Use caution in patients receiving concomitant beta-blockers (e.g., atenolol [Tenormin], propranolol), calcium channel blockers (e.g., diltiazem [Cardizem], verapamil), or any agents used for fluid and electrolyte balance. Will require adjustment in these therapies as symptoms are controlled by octreotide. ■ May affect absorption of orally administered medications. ■ May inhibit effectiveness of cyclosporine and may result in transplant rejection. ■ Markedly increases adsorption of insulin and reduces availability. ■ Concurrent use with oral antidiabetic agents, glucagon, growth hormone, or insulin may cause hypoglycemia or hyperglycemia. Monitor patient carefully and give adjunct dose of these agents as indicated. ■ May increase availability of bromocriptine (Parlodel). ■ Suppression of growth hormones may cause a decreased clearance of drugs metabolized by selected cytochrome P450 enzymes. Use caution with concurrent use of drugs metabolized by CYP3A4 (e.g., cisapride [Propulsid], erythromycin, HMG-CoA reductase inhibitors [lovastatin (Mevacor) and simvastatin (Zocor)], itraconazole [Sporanox], oral midazolam [Versed], quinidine, terfenadine).
Side effects
Most side effects are of mild to moderate severity and of short duration. Abdominal pain/discomfort, abnormal stools, anorexia, anxiety, biliary sludge, cholelithiasis, constipation, convulsions, depression, diarrhea, dizziness, drowsiness, fat malabsorption, fatigue, flatulence, fluttering sensation, GI bleeding, headache, heartburn, hepatitis, hyperesthesia, hyperglycemia, hypoglycemia, increase in liver enzymes, insomnia, irritability, jaundice, nausea, pancreatitis, pounding in the head, rectal spasm, swollen stomach, vomiting. In rare cases, GI side effects may resemble intestinal obstruction with progressive abdominal distension, severe epigastric pain, abdominal tenderness, and guarding. Many other side effects occur in fewer than 1% of patients. Side effects that occur more often in patients with acromegaly include cardiac abnormalities (e.g., sinus bradycardia, ECG changes [including QT prolongation], conduction abnormalities, and arrhythmias) and hypothyroidism.
Post-marketing:
Intestinal obstruction, thrombocytopenia.
Antidote
Keep physician informed of all side effects. A dose adjustment of either octreotide or other concomitant therapies may be required. Symptomatic and supportive treatment may be indicated. Overdose will cause hyperglycemia or hypoglycemia depending on tumor involved and endocrine status of patient. Discontinue octreotide temporarily, notify the physician, and monitor the patient carefully. Symptomatic treatment should be sufficient.
Ofatumumab 
(oh-FAT-oo-moo-mab)
Arzerra
Monoclonal antibody
Antineoplastic
pH 5.5
Usual dose
Premedication:
Patients should receive all of the following premedication agents 30 minutes to 2 hours before each infusion of ofatumumab. The premedication schedule is listed in the following chart.
| Premedication Schedule for Ofatumumab | |||||
| Previously Untreated CLL or Extended Treatment of CLL | Refractory CLL | ||||
| Infusion number | 1 and 2 | 3 and beyond* | 1, 2, and 9 | 3 to 8 | 10 to 12 |
| IV corticosteroid (prednisolone or equivalent) | 50 mg | 0 to 50 mg† | 100 mg | 0 to 100 mg† | 50 to 100 mg‡ |
| Oral acetaminophen | 1,000 mg | ||||
| Oral or IV antihistamine | Diphenhydramine 50 mg or cetirizine 10 mg (or equivalent) | ||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


