CHAPTER 31 Nursing the patient with cancer
Introduction
Despite improvements in survival, the term ‘cancer’ impacts on the attitudes and beliefs of practitioners and patients, instilling thoughts about pain, uncertainty and death (Box 31.1). Government policy in prevention and predictions about the nature and cause of cancer can appear controversial and contradictory. This creates misinformation and inconsistency surrounding the disease.
Box 31.1 Reflection
![]() See website for further content
See website for further content
Cancer is a serious social problem, costing much in human and financial terms. One in three people in the UK risk developing cancer in their lifetime. One in four deaths are attributable to cancer (Cancer Research UK 2009). The incidence of different cancers and the death rate associated with them can vary both within the UK and globally. (See Useful websites Cancer Research UK, World Health Organization.)
Oncology as a specialty
The UK political and health care agenda has recognised cancer as a high priority area for over a decade. The first important documentation to drive organisational change in cancer services across the country was initiated by the publication of the Calman–Hine/Calman Report (Department of Health 1995). The Calman Report focused around the patient and their family, care being delivered in specific designated cancer centres and cancer units in partnership with each other. The report recommended a seamless service integrating primary, secondary and tertiary care, with real commitment to psychosocial as well as medical needs. It also provided the opportunity to think about cancer control, early screening and detection programmes.
Subsequent policy documents have influenced the progression of cancer services and, following devolution, England, Wales and Scotland have set out their own policy directions for health (Department of Health 2004, Scottish Government 2008). Comprehensive long-term strategies are continually being developed to shape cancer services, focusing on all aspects of the patient’s cancer journey, ensure equity of care and invest in equipment and the cancer workforce. Nursing plays a key role in this development and both student nurses and newly qualified health care professionals need to understand the multidisciplinary nature of cancer management within and outside the hospital, appreciating the roles of all those providing patient care.
The fundamental process of cancer
In recent decades, molecular biology has made great progress in unravelling the steps involved in this process of cell transformation and is improving our ability to understand, classify, diagnose and treat cancer. A critical component in understanding the cancer process and rationale for treatment is a knowledge of the structure and function of cells, the role of deoxyribonucleic acid (DNA) and the cell cycle. (See Further reading, e.g. Martini & Nath 2009 and Useful websites, e.g. Cancerquest.)
Tumour pathology
Once removed, benign tumours rarely recur. However, a benign tumour can cause problems or even death in certain sites. For example, a benign brain tumour can cause pressure on the brain (see Ch. 9) and some benign tumours cause problems in other ways, such as bleeding or secreting excess hormones.
Malignant tumours grow at the expense of the host. They are characterised by:
Understanding the molecular biology of cancer is very important to identify and obtain a definitive cancer diagnosis and thus predict prognosis. Increasing knowledge about newer biological factors such as HER2/neu receptors and proliferative index (rate of cell division in the tumour) has altered the way adjuvant treatments (see p. 812) are prescribed and these indicators are increasingly used to predict prognosis. The HER-2/neu receptor belongs to the epidermal growth factor receptor family which is one of the receptors critical in controlling the pathway for epithelial cell growth and differentiation, and possibly angiogenesis (Karunagaran et al 1996). Overexpression of the HER-2/neu protein is observed in 15–20% of breast cancers (Slamon et al 1989), and it is now accepted that high levels of expression of HER-2/neu identify those patients most likely to respond to trastuzumab in both the adjuvant and metastatic disease setting.
Identification of the steps involved in carcinogenesis has led directly to the discovery of molecular tumour markers (Table 31.1). Among the cancers for which molecular diagnostics has had an impact is chronic myeloid leukaemia (CML). The marker of this disease is the Philadelphia chromosome, which is detectable in 95% of cases. This additional information enhances the ability to accurately diagnose and determine prognosis.
Table 31.1 Examples of molecular genetic markers used in cancer diagnosis and prognosis
| Cancer | Genetic Marker | Principal Application |
|---|---|---|
| Chronic myeloid leukaemia | Philadelphia chromosome t(9;22)(q34;q11) [BCR/ABL] | Primary diagnosis, detection of residual disease after treatment |
| Non-Hodgkin’s lymphoma | ||
| Follicular | t(14;18)(q32;q21) [BCL2/IGH] | Primary diagnosis, detection of residual disease after treatment |
| Burkitt’s | t(8;14)(q24;q32) | As above |
| Neuroblastoma | MYCN amplification | Prognosis |
| Breast cancer | HER2-neu/ERB2 amplification | Prognosis |
| Familial cancers | ||
| Breast | BRCA1, BRCA2 | Diagnosis of hereditary predisposition |
| Colon | APC, MSH2, MLH1 | As above |
| Wilms’ tumour | TP53 mutation | As above |
| Retinoblastoma | RB mutation | As above |
Tumours may be classified not only by their biological behaviour but also traditionally by their tissue of origin. Most tumours retain sufficient characteristics of the normal differentiated cell to allow recognition of the type of tissue from which they were derived, which is the basis for the classification of tumours by tissue type (Table 31.2).
Table 31.2 Classification by tissue type of malignant tumours
| Tissue of Origin | Malignant Tumour |
|---|---|
| Epithelial | ‘Carcinoma’ |
| Squamous: surface epithelium, cell lining covering body cavities, organs and tracts | Squamous cell carcinoma, e.g. lung, skin, stomach |
| Glandular: glands or ducts in the epithelium | Adenocarcinoma, e.g. breast, lung, colon |
| Transitional cells: bladder lining | Transitional cell carcinoma, e.g. bladder |
| Basal cells: skin layer | Basal cell carcinoma (BCC), ‘rodent ulcer’ |
| Liver | Hepatocellular carcinoma |
| Biliary tree | Cholangiocarcinoma |
| Placenta | Choriocarcinoma |
| Testicular epithelium | Seminoma, teratoma, embryonal carcinoma |
| Endothelial cells | Angiosarcoma |
| Mesothelial: covering the surface of serous membranes | Mesothelioma, e.g. pleura, peritoneum |
| Connective tissue | ‘Sarcoma’ |
|---|---|
| Bone | Osteosarcoma |
| Cartilage | Chondrosarcoma |
| Fatty tissue | Liposarcoma |
| Fibrous tissue | Fibrosarcoma |
| Lymphoid tissue | Lymphomas |
| Bone marrow | Leukaemias, e.g. ALL, CML |
| Muscle | ‘Myosarcoma’ |
|---|---|
| Smooth muscle | Leiomyosarcoma |
| Striated muscle | Rhabdomyosarcoma |
| Cardiac muscle | Cardiac sarcomas |
| Neural | |
|---|---|
| Meninges | Meningeal sarcoma |
| Glia | Glioblastoma multiforme |
| Neurones | Neuroblastoma, medulloblastoma |
| Germ cells | |
|---|---|
| Testes or ovary | Teratoma, germ cell |
ALL, acute lymphoblastic leukaemia; CML, chronic myeloid leukaemia.
The physical effects of cancer
Direct tumour effects
Cancer recurrence
The exact manner in which a cancer moves from being a carcinoma in situ to one which is capable of invasion and spread to other organs or tissues is not clear; however, it is thought to be partly due to physiological changes occurring in tumour cell membranes which reduce their adhesion to other cells. Tumours also produce proteolytic (protein-dissolving) enzymes, which may assist in the invasion of normal tissue. Malignant cells also seem to lose contact inhibition, failing to recognise their boundaries and to cease growth on meeting a different tissue type. For example, tumours of glandular lung tissue may continue invasion through the pleura to the chest wall. The risk of cancer recurrence is always present when a diagnosis has been given. Two thirds of patients develop metastases and most deaths from cancer are due to metastases that are resistant to conventional therapies (Fidler 1997). (See Useful websites, e.g. Cancerquest; tumour biology.)
Metastatic invasion (spread) may occur in one of four ways (see Figure 31.1):
The psychosocial impact of cancer
There are psychosocial implications for patients and families from diagnosis, through treatment, the development of recurrence and end of life. It is now widely accepted that quality of life in cancer patients is an important consideration and may be improved by psychological interventions at specific stages (Cruickshank et al 2008). These important areas are explored further in this chapter.
Epidemiology of cancer
Epidemiological studies provide data about possible causes of different forms of cancer and inform the strategies for cancer prevention and screening. The most commonly diagnosed cancers in the UK are breast, lung, colorectal and prostate; together these four cancers make up over 50% of all new cancers each year (Cancer Research UK 2009). The 20 most commonly diagnosed cancers are shown in Figure 31.2.
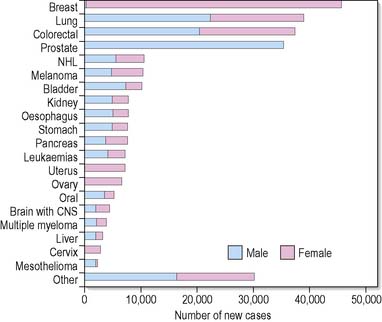
Figure 31.2 The 20 most commonly diagnosed cancers (ex NMSC), UK, 2006 (Cancer Research UK, http://info.cancerresearchuk.org/cancerstats/incidence/commoncancers/ June 2009). CNS, central nervous system; NHL, non-Hodgkin’s lymphoma.
Cancer is associated with increasing age. Around 75% of people diagnosed with cancer are aged 60 years and over (Cancer Research UK 2009). Cancer is very rare in children; approximately 1% of cancers are diagnosed in children, teenagers and young adults (Cancer Research UK 2009). (See Further reading, e.g. Baggott et al 2002.)
The incidence of cancer in the UK is one of the highest in the northern hemisphere. Predominantly, cancer is a disease of well-resourced countries but it is clear that patterns of cancer are set to increase in developing countries over the next decade (Ferlay et al 2007). The greater incidence in the northern hemisphere may be partly attributed to the availability of screening, diagnostic and reporting procedures, improved overall life expectancy and diet. Geographical variations across the world in the incidence of cancer provide clues to the lifestyles of different populations.
Aetiology of cancer
Carcinogenic factors and substances can be either intrinsic or extrinsic.
Intrinsic factors
Heredity
Cancer susceptibility genes such as BRCA1 and BRCA2 have been identified in breast and ovarian cancer. Women who inherit a mutation in these genes may face a 50–85% lifetime risk of developing breast cancer including an increased risk for ovarian cancer (Calzone & Biesecker 2002). Testing for these particular genes and others is now available commercially.
Hormones
Certain hormones are thought to promote some tumours. Early menarche, late menopause and nulliparity are associated with an increased lifetime risk of breast cancer. The Million Women Study (2003) found that the use of combined oestrogen plus progesterone hormone replacement therapy (HRT) also increases the risk of breast cancer. High testosterone levels have been related to increased risk of prostate cancer (Ross et al 2003).
Immunity
Individuals with impaired immunity are more susceptible to cancer; for example, people with HIV disease have a higher incidence of Kaposi’s sarcoma than the normal population (see Ch. 35). It is the prolonged immunosuppression that may predispose these patients to cancer rather than the infectious agent per se.
Extrinsic factors
Physical agents
Radiation
is known to cause cellular mutations and cancer. Survivors of the atomic bomb explosions in Hiroshima and Nagasaki in Japan experienced a high incidence of leukaemia and skin cancer. Early research has shown an apparent increase in the incidence of leukaemia among the children of fathers working in the nuclear industry, possibly due to germ cell mutations (Gardner et al 1990). Repeated exposure to therapeutic doses of radiation is not thought to be harmful, although stringent precautionary regulations must be followed for the protection of all exposed workers. Therefore, it is not routine discharge of radioactivity by the nuclear industry that should be feared, but rather the catastrophic event, such as occurred at Chernobyl in 1986.
Ultraviolet light (UVL)
There is overwhelming evidence that repeated exposure to solar UVL is the primary cause of basal and squamous cell carcinoma (see Ch. 12). Data establishing a direct causal relationship with sunlight are more complex, but suggest a promotional role of sunlight in the cause of melanoma. Light-skinned or freckled individuals who are unable to manufacture sufficient protective melanin are particularly at risk (see Ch. 12).
Chemical agents
Tobacco
Ninety per cent of all lung cancers can be attributed to tobacco smoking. The temporal relationship between smoking and lung cancer was defined in the 1950s by studies undertaken by Doll & Hill (1954). Cigarette smoking has now clearly been identified as a major cause of cancers of the mouth, pharynx, larynx, bladder and pancreas, whilst contributing to many others. Risk is more dependent on duration of smoking than on consumption. Smoking 20 cigarettes a day for 40 years is eight times more hazardous than smoking 40 cigarettes a day for 20 years. People who stop smoking even well into middle age avoid most of their subsequent risk of lung cancer, and stopping before middle age avoids more than 90% of the risk attributable to tobacco (Peto et al 2000). It is also evident that non-smokers are at risk from exposure to other people’s smoke: one-quarter of lung cancer cases in non-smokers are estimated to be due to passive smoking (International Agency for Research on Cancer [IARC] 2004).
Viruses
Although cancer is not contagious, some cancers are associated with viral infections. For example, the Epstein–Barr virus (EBV) causes a systemic infection that may precede Burkitt’s lymphoma, a malignant disease common in parts of Africa. Patients with chronic hepatitis B are more susceptible than others to liver cancer. The human papilloma virus (HPV), which may be sexually transmitted, is associated with cervical cancer, and the development of vaccines against some types of HPV has created opportunities to prevent some cases of cervical cancer (see p. 812).
Cancer prevention and screening
Primary prevention
Primary cancer prevention includes activities such as:
Health promotion programmes
Health promotion/education programmes have some measure of success. Several government publications have taken on ‘The Cancer Challenge’, with measures to promote a ‘pro-health’ culture (Department of Health 2000, Scottish Executive Health Department [SEHD] 2004). Projects have been developed to assist smokers to quit, particularly in areas with high levels of deprivation, prisons, the army and working men’s clubs. Political action has included a comprehensive ban on tobacco advertising, a smoking ban in public places and the workplace, the setting up of new stop-smoking clinics and a smokers’ helpline, nicotine replacement patches and bupropion available on prescription, as well as a major health education campaign in schools aimed at preventing youngsters from starting to smoke (Department of Health 2000, SEHD 2004).
Through working partnerships with the community and the food industry, strategies to promote healthy eating include a national ‘Five a Day’ programme, improving accessibility to affordable fresh fruit and vegetables, and advocating five portions of these daily (see Ch. 21) (Box 31.2).
Attitudes
Psychologists have developed a Health Belief Model (Strecher & Rosenstock 1997) to predict an individual’s preventive health behaviour and account for some of the factors which determine attitudes to health and illness. The Health Belief Model states that an individual feels vulnerable to a disease if they believe they are susceptible to developing it and believe the disease to be serious. Preventive action will be taken only after the individual has balanced the benefits of that action against its physical, psychological and financial costs (Smith 2005).
Secondary prevention: screening
Cancer screening difficulties
It must be possible to identify an at-risk group; otherwise the cost of screening becomes prohibitive. Finally, and most problematically, it must be determined whether detecting the cancer type at an early stage will prolong life (Segnan et al 2004).
The general cancer screening programmes currently offered (in the UK) are:
Plus the Prostate Cancer Risk Management.
Breast cancer screening
Despite recent debates about the quality of over 40 years of trials, it is generally agreed that there is a clear benefit and reduction in mortality from breast cancer from screening women over the age of 50 years by mammography every 2 years (IARC 2002). In the UK mammography is routinely offered every 3 years to women aged between 50 and 70 years. However, the age range for routine invitation for screening is to be extended to include women aged 47 to 73. Accepted screening techniques include mammography and clinical breast examination, although Baxter (2001) has concluded that breast self-examination is of no benefit in routine screening. The value of screening women of 40 years or younger remains controversial, consequently professional clinical judgement and a woman’s choice should guide decision-making. The denser breast tissue of premenopausal women makes mammograms difficult to interpret in this age group. In future, genetic screening and new technologies such as digital mammography may be of benefit.
Cervical cancer screening
Uptake of the service by groups at highest risk, i.e. women over the age of 40, of lower socioeconomic status and in minority ethnic groups, could be better. Barriers to cervical screening include lack of sensitivity and trust in health professionals, possible feelings of guilt and embarrassment, judgemental attitudes, and a lack of privacy and supportive care in clinics (Fitch et al 1998). It is important for staff working in screening services to have well-developed interpersonal and communication skills to alleviate any fears or anxieties. In addition, clinic schedules should allow time to provide support.
Intervention strategies based on individual respect, health care provider relationships and inclusion of significant others may increase adherence to cancer screening guidelines (Steven et al 2004).
Colorectal cancer screening
Screening is routinely offered every two years to people aged 60–69 years (in England) and 50–74 years (in Scotland). However, people aged over 70 years in England can request a screening kit (NHS Cancer Screening Programme 2009a). People with an unclear result will have the FOB test repeated. Where an abnormal result occurs the person is offered an appointment with a specialist nurse to discuss colonoscopy (see Ch. 4). Campaigns by Colon Cancer Concern have been initiated to promote a greater awareness of the early warning signs of colon cancer (see Ch. 4).
Screening options for other cancers
Screening programmes for the cancers below are being reviewed, including:
Prostate cancer
There is at present no consensus regarding the most appropriate screening method. There are three main screening modalities: digital rectal examination (DRE), serum prostate-specific antigen (PSA) and transrectal ultrasonography (TRUS). There are wide ranges in the estimates of sensitivity and specificity. Interest in PSA (a blood test) emerged in the late 1980s. However, PSA may be elevated in men with non-cancerous conditions. There is currently no evidence that prostatic screening improves clinical outcomes; in fact, there are issues of uncertainty surrounding the appropriateness and type of treatment for men with early-stage prostatic cancer, since it has a long asymptomatic latency period. It is therefore important to consider that there might be an adverse psychological impact as a result of prostate screening although, for those men at risk, it may provide some reassurance (Cantor et al 2002).
A Prostate Cancer Risk Management programme is available to improve men’s understanding of the benefits and limitations of PSA testing (NHS Cancer Screening Programme 2009b).
Ovarian cancer
This cancer is sometimes referred to as the ‘silent killer’ because it often presents late and has the highest mortality rate of all malignant gynaecological cancers. Transvaginal ultrasound and detection of a raised cancer antigen (CA125) in the blood provide two possible techniques for the screening of ovarian cancer. However, as with PSA, CA125 may be elevated in non-malignant conditions. A UK collaborative trial of ovarian cancer screening (UKCTOCS) is underway to answer the question of whether or not early detection will save lives. The effectiveness of different screening technologies is being examined. A report is expected in 2010 and will make recommendations about ovarian cancer screening for the whole population (Menon et al 2009).
Lung cancer
It has been shown that low-dose spiral computed tomography (CT) scanning can identify lung cancer in high-risk but asymptomatic individuals (Gohagan et al 2005). Whilst this may be a useful screening test, further evidence is required to see if early detection is linked with a decrease in mortality.
Medical intervention and the nurse’s role
Diagnosis and staging
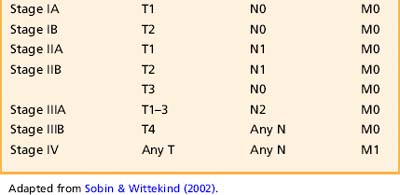
The tumour, node (lymph) and metastasis (TNM) system
The most common internationally used method of defining disease stage is the TNM system (Sobin & Wittekind 2002), in which:
Agreed staging criteria exist for each cancer, such as those staging systems used for lung cancer (Box 31.3), gynaecological, testicular cancers, etc. (see Chs 3, 7). Box 31.4 illustrates the staging system through the experience of a young man with testicular cancer.
Box 31.3 Information
Adapted from Sobin & Wittekind (2002).
Summary of the TNM staging system for lung cancer
T (primary tumour)
| TX | Positive cytology only |
| Tis | Carcinoma in situ |
| T1 | Tumour ≤3 cm in diameter. No proximal invasion |
| T2 | Tumour >3 cm in diameter, within 2 cm from carina or invading the visceral pleura or partial atelectasis |
| T3 | Tumour of any size extending into the chest wall, diaphragm, pericardium, mediastinal pleura or within 2 cm of carina, total atelectasis |
| T4 | Tumour of any size with invasion of mediastinal organs or vertebral body, malignant pleural effusion |
N (lymph nodes)
| N0 | Nodes negative |
| N1 | Positive nodes in ipsilateral hilar nodes |
| N2 | Positive ipsilateral, mediastinal and subcarinal nodes |
| N3 | Positive contralateral mediastinal or hilar nodes, scalene or supraclavicular nodes |
Box 31.4 Reflection
Staging Dan’s testicular cancer
Staging system generally used in the UK
| Stage I | Tumour confined to testes |
| Stage II | Pelvic and abdominal lymph node involvement |
| Stage III | Mediastinal and/or supraclavicular lymph node involvement |
| Stage IV | Distant metastases, e.g. lung |
Psychological impact of diagnosis and staging
Primary diagnosis
It is very common for patients to be aware that they have cancer before they are told formally of their diagnosis. This awareness derives from their experience of symptoms, tests and, in some cases, surgery and from the non-verbal communication of staff or relatives. How to inform patients in full of their diagnosis, and when this should be done are ongoing issues of ethical debate (Box 31.5).
Box 31.5 Information
Informing patients of their diagnosis: ethical considerations
Two ethical principles are central to the discussion of whether it is always right to tell a patient the whole truth about their diagnosis: these are the principles of autonomy and of beneficence (see further reading, e.g. Thompson et al 2006).
Autonomy
Patients have a right to autonomy, or self-determination. They cannot make decisions about their treatment or the future if they are not fully aware of their diagnosis. However, research has suggested that denial as a coping mechanism is sometimes necessary for the preservation of well-being during a crisis, allowing an individual time to mobilise the resources to cope with the seriousness of their disease (Moyer & Levine 1998). Unlike years ago, patients are now normally told their diagnosis, with a discussion of prognosis – often a medical uncertainty – and the level of information is tailored to the individual’s needs. There is no evidence to suggest that acceptance correlates positively to survival in cancer (Spiegel 2001).
Discussion
In most cancer centres, the issue is not whether, but how and when, to inform patients of diagnosis and prognosis. A study by Schofield et al (2003) showed that clinical practices that are linked to lessening anxiety include preparing the patient for a possible cancer diagnosis, having significant others present at diagnosis, being given as much information as desired in understandable language, having questions answered, talking about feelings and being given reassurance. It would seem appropriate that a nurse is present at such discussions because they can follow up the conversation and help the patient to strike a balance between realistic hope and the acceptance of reality.
Box 31.6 provides an opportunity for you to reflect on patient experiences of being given a diagnosis of cancer.
Box 31.6 Reflection
Psychological support
The nurse has an important role providing both practical information prior to and following each test, and emotional support. It is important to know why each test is being performed and what it will entail for the patient. Findings from research suggest that a substantial proportion of the lay public do not understand phrases used in cancer consultations. One study found that only 52% understood that the phrase ‘the tumour is progressing’ was not good news and less than a third understood what was meant by ‘seedlings’ (Chapman et al 2003). The nurse, acting as patient advocate, should ensure they are present when doctors explains test results so they are able to offer additional support and revisit this information again with the patient if necessary. The patient may have fears they wish to express and an opportunity to discuss these openly is important. The nurse is well placed do this.