Gastrostomy and Jejunostomy Tubes
Comparative Complication Rates
Percutaneous tube placement into body organs or spaces is a means for drainage of fluids, maintaining an opening into an organ where obstruction exists, or providing for instillation of fluids, medication, or feeding through the tube. The tubes are usually placed by a physician in surgery, via endoscopy or interventional radiology. The WOC nurse is often consulted for management of these tubes and the complications that may occur with them. Knowledge of the location, purpose, and desired outcome of the tube placement is essential to effectively manage the care of patients with these tubes. The use of percutaneous tubes is common in the adult and pediatric patient populations across acute care, long-term care, and home care settings. Increasingly they are being used for pain relief and symptom management in palliative care (Requarth, 2011). Common types of these tubes are gastrostomy, jejunostomy, biliary, and nephrostomy.
Gastrostomy and Jejunostomy Tubes
Nasogastric tubes (NGT) are the simplest to insert in the gastrointestinal (GI) tract and the least invasive, but they carry a higher risk for dislodgment and aspiration leading to pneumonia (Hsu et al., 2009). When feeding through the tube or decompression of the GI tract is needed for more than a few weeks, a percutaneous tube is inserted. NGT are indicated for short-term use. Common indications for gastrostomy tube (GT) insertion are obstructing head and neck cancer, benign and malignant esophageal disease, neurologic dysfunction, trauma, and respiratory failure.
GTs have been reported in the literature since the 1800s. Dr. Martin Stamm developed a surgical procedure for placement of a tube directly into the stomach, which is still used today. The standard Stamm gastrostomy involves circumferential purse-string sutures to stabilize the tube within the lumen of the stomach and affix the stomach to the anterior abdominal wall. A later technique developed by Witzel involves creating a serosal tunnel as well as an abdominal wall tunnel through which the tube passes. This is useful when the stomach has been altered so that it cannot be secured to the abdominal wall such as after a gastric bypass surgery or resection of esophageal cancer (Gaurav, 2014). Variations on these open surgical procedures remained the standard of care for feeding or gastric decompression until the 1980s when a procedure for percutaneous endoscopic gastrostomy (PEG) was developed. PEG is a method of placing a tube into the stomach through the skin, aided by endoscopy (Fig. 18-1). A PEG with a jejunal extension tube can be placed through a preexisting PEG to facilitate more distal feeding while also providing an avenue for gastric decompression when necessary.
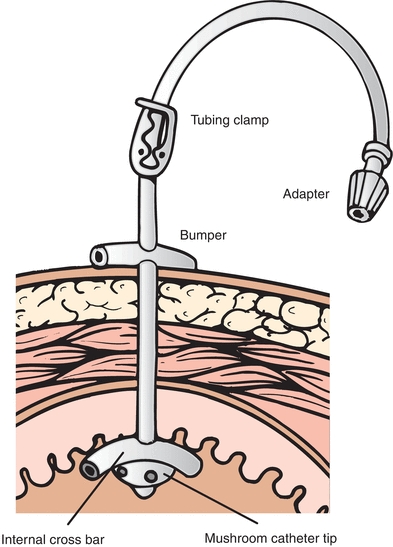
FIGURE 18-1. PEG tube with internal and external bumper.
PEG tube placement is one of the most common endoscopic procedures performed today, and an estimated 100,000 to 125,000 are performed annually in the United States (Gaurav, 2014).
PEG is now considered the method of choice for enteral access due to the simplicity, effectiveness, and lower cost of the procedure (Miller et al., 2014). However, PEG is not always clinically appropriate, and some of the possible contraindications include the following:
- Uncorrected coagulopathy or thrombocytopenia
- Upper tract obstruction or malformation
- Severe ascites
- Hemodynamic instability
- Sepsis
- Intra-abdominal perforation
- Active peritonitis
- Abdominal wall infection at the selected site of placement
- Gastric outlet obstruction (if PEG tube is being placed for feeding)
- Severe gastroparesis (if PEG tube is being placed for feeding)
- History of total gastrectomy
When a PEG is not feasible for the patient, radiologic placement is a possible alternative. This was first described in the literature in the mid-1980s (Duszak, 2014) and avoids the use of an endoscope and is not contraindicated in the presence of upper tract obstruction. It uses fluoroscopy and ultrasound to identify the stomach, and a GT with a balloon is secured against the gastric mucosa with an external bumper on the skin (Fig. 18-2). If gastroesophageal reflux or delaying gastric emptying is a problem, another feeding tube option is a percutaneous gastrojejunal tube (Fig. 18-3). This tube has a balloon and an external skin bumper. There is an extension that is guided through the duodenum and into the jejunum for feeding. These tubes will have a gastric port that can be used for medication or fluid administration or decompression of the stomach and one for the jejunal feeding. A study of 124 patients requiring conversion from a GT to gastrojejunal tube showed a significantly higher success rate using the radiologic placement procedure rather than nonradiologic procedures (Kim et al., 2010). These radiologic procedures require providers with training in interventional radiology, which is not always an option in all facilities.

FIGURE 18-2. Balloon-tipped gastrostomy tube.
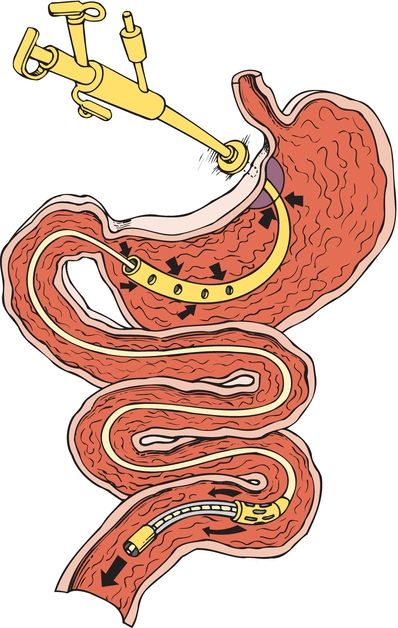
FIGURE 18-3. PEG with jejunal extension.
Both the PEG and radiologic procedures can be done with sedation rather than anesthesia making the procedure safer for the patient, and the time for initial feedings is not delayed. If feeding in the stomach is not possible due to surgical absence of the organ, severe gastroparesis, or gastric outlet obstruction, radiologic intervention is used to place a feeding tube directly into the jejunum. This tube is secured with a stabilizer sutured to the skin.
For those patients who are not candidates for PEG or radiologic procedures, surgical approaches offer the advantage of direct visualization of tube placement into the intended organ (stomach or jejunum). An open laparotomy or laparoscopy is done by a surgeon in the operating room, and the patient receives general anesthesia. During the surgical approach, the stomach or jejunum is identified following a laparotomy incision or insertion of the laparoscope. The laparoscopic approach offers smaller incision size, less pain, and decreased risk of incisional hernia (Mizrahi et al., 2014). The appropriate feeding tube is secured within the lumen of the targeted organ and brought out through a separate stab incision. If a patient is scheduled for an open or laparoscopic abdominal surgery and it is expected that a feeding tube may be needed, it should be placed at the time of the surgery.
Comparative Complication Rates
There are many potential complications with these procedures, and most of the patients are malnourished and have significant comorbidities. However, the complication rates are relatively low. The complication rates reported in the literature vary, but it seems generally accepted to be 1% to 3% for PEG placement, 8% to 10% with radiologic procedures, and 7% to 15% with surgical procedures (Miller, 2014). Complications associated with percutaneous endoscopic approaches include endoscopic trauma and perforation of the GI tract, bleeding, skin and soft tissue infection, injury to intra-abdominal viscera such as the liver or colon, tube dislodgment, and fistula creation. Radiologic placement has many of the same risks as do endoscopically placed tubes, but there is no risk of upper tract trauma from the endoscope. Surgically placed tubes are associated most commonly with skin and soft tissue infection, incisional hernia, bleeding, inadvertent removal of the tube, and complications associated with general anesthesia. Issues with inadvertent injury to surrounding intra-abdominal viscera are very rare due to the better visibility during the procedure. In a study comparing laparoscopic versus open laparotomy, the laparoscopic surgery took longer to perform, but the complication rate was higher in the open surgery group (Mizrahi et al., 2014).
Routine Tube Care
Following placement of percutaneous tubes, the external bolster should generally be left in place for at least 4 days. After 4 days, there should be 1/2 to 1 cm of laxity left between the entry point and the bumper of the tube to prevent ulceration of the gastric mucosa or pressure damage to the skin under the bumper. Due to the possibility of edema at the tube site, positioning of the tube should be observed frequently for the first 48 hours after insertion (Miller et al., 2014). Evidence for most effective site care is lacking, but patient education materials recommend that the site be washed with mild soap and water, rinsed well with water, and dried daily. One gauze drain sponge may be placed under the bumper unless it sutured in place to absorb any drainage from the site. The use of a dressing after the first week is optional if there is no drainage around the tube site. It is important to know how much fluid was put in the balloon at the time of placement (if the tube is a balloon tube) and what manufacturer made the tube. The manufacturer’s Web sites have specific information about their tubes and recommendations about how often to check the fluid levels in the balloon. If leakage is a problem, check the balloon for fluid and refill the balloon to the level placed at the time of the tube insertion. Use sterile water to fill the balloon (Simons, 2013).
CLINICAL PEARL
If there is crusting around the opening, use a water-moistened cotton-tipped applicator to gently remove.
BOX 18-1 Procedure for Pouch Application around the Gastrostomy Tube
Equipment:
Ostomy pouch
Scissors
Skin barrier powder
Wet and dry cloths
No sting skin prep
Cotton-tipped applicators
Gloves
Catheter holder device
Water-resistant tape
Directions:
1. Clamp tube and turn off feeding.
2. Remove the pink tape from around the tube where it exits the pouching system and gently remove the pouch using adhesive remover or warm water. Be careful not to pull or dislodge the tube.
3. Clean the skin with water and pat dry. Cleanse the area under the tube bumper by inserting a cotton-tipped applicator between sutures. Sprinkle skin barrier powder under the bumper to protect the skin.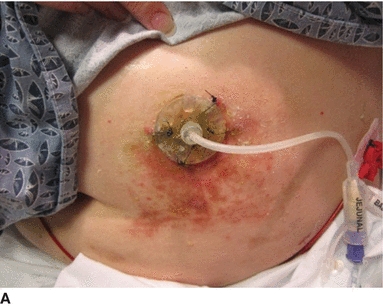
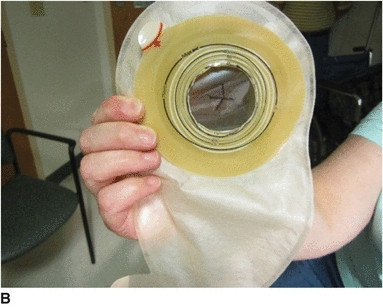
4. If there is any skin breakdown (A), sprinkle skin barrier powder on the skin, rub in, and seal with no-sting liquid skin barrier.
5. Cut an opening in skin barrier of the one-piece pouching system to fit around the bumper. Cut an X-shaped opening on the front of the pouch so the gastrostomy tube can be pulled through (B).
6. Place catheter holder device over X cut in front of the pouch to secure the tube and avoid leakage (C). Instructions come with each device. Cut a hole in the nipple large enough to pull the tube through (D).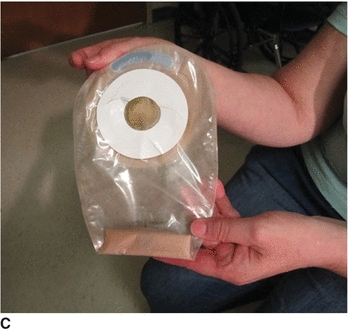

7. Pull tube through the opening and place the pouch on the skin. Make sure the skin is dry before placing the pouch (E). Use pink tape around the tube where it exits the pouch to seal the opening in the nipple (F).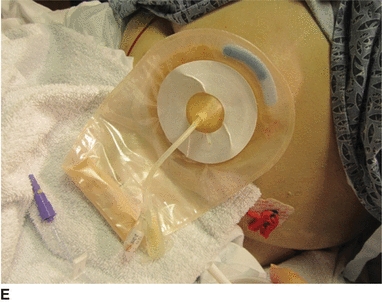
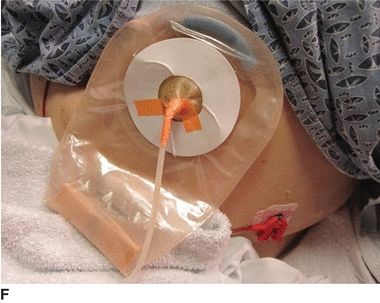
The time before the tube can be used for feeding varies with the procedure performed and the preference of the provider. When feeding is allowed, it is important to routinely flush the tube to prevent clogging from occurring. It should be flushed with 30 mL water before and after each feeding and every 4 to 6 hours when the patient has continuous feedings. Use 10 mL water to flush before and after giving each medication. If liquid medication is not available, the medication should be finely crushed and mixed with water (Simons & Remington, 2013). If the tube does become clogged, try the following:
- Be sure the tube is not kinked.
- Milk the tube to remove any mechanical obstruction.
- Aspirate any fluid from the tube and then instill 10 mL warm water with a 60-mL catheter tip syringe and pull back and forth on the plunger to try to dislodge the obstruction.
- If it is still clogged, repeat the above step with one pancreatic enzyme tablet and one sodium bicarbonate tablet crushed and mixed in 5 mL of water (WOCN, 2008).
- If the tube cannot be unclogged, contact the physician. Instrumentation or replacement may be necessary.
Managing Skin Complications
There are many types of possible complications with enteral feeding tubes. The most serious adverse effects, such as abscess or necrotizing infection in the skin around the tube; buried bumper syndrome, where the internal tube bumper becomes imbedded in the gastric mucosa; or hemorrhage at the tube site, are uncommon. Complications that may require a consult for the WOC nurse are those that involve skin breakdown (Table 18-1). The most common cause of skin breakdown around the tube site is leakage of gastric contents on the skin. This is caused by movement of the tube that may enlarge the opening in the skin. To stabilize the tube, gently pull up on the tube until the internal anchoring device (bumper) or balloon is against the wall of the stomach and then slide the external stabilizer down to rest comfortably on the skin without excess tension (WOCN, 2008). If there is an external stabilizer sutured to the skin with a jejunostomy and the sutures are no longer intact, it may be necessary to have these replaced. This tube is not secured with an internal bumper or a balloon, so it will migrate if sutures are not present. If there are sutures in the bumper of a PEG tube stabilizer, they may impede the ability to care for the skin and prevent skin complications such as irritant dermatitis and device-related pressure ulcers. It is appropriate to ask if these can be removed after healing has taken place. If there is no external bumper, the use of a commercial stabilizing device to secure the tube (Fig. 18-4) or taping the tube in place may prevent movement. With a balloon-tipped tube, loss of water in the balloon will cause migration of the tube. Replacing a leaking tube with a larger diameter tube in the hopes of obtaining a better seal is not effective and is contraindicated (Stayner et al., 2012). This will further enlarge and distort the leaking tube tract. In rare cases of persistent leakage, the tube must be removed and placed in a different site allowing the original site to close.
CLINICAL PEARL
It is recommended that the amount of water in the balloon is checked weekly and replaced with the correct amount.
TABLE 18-1 Complications Associated with Enteral Tubes
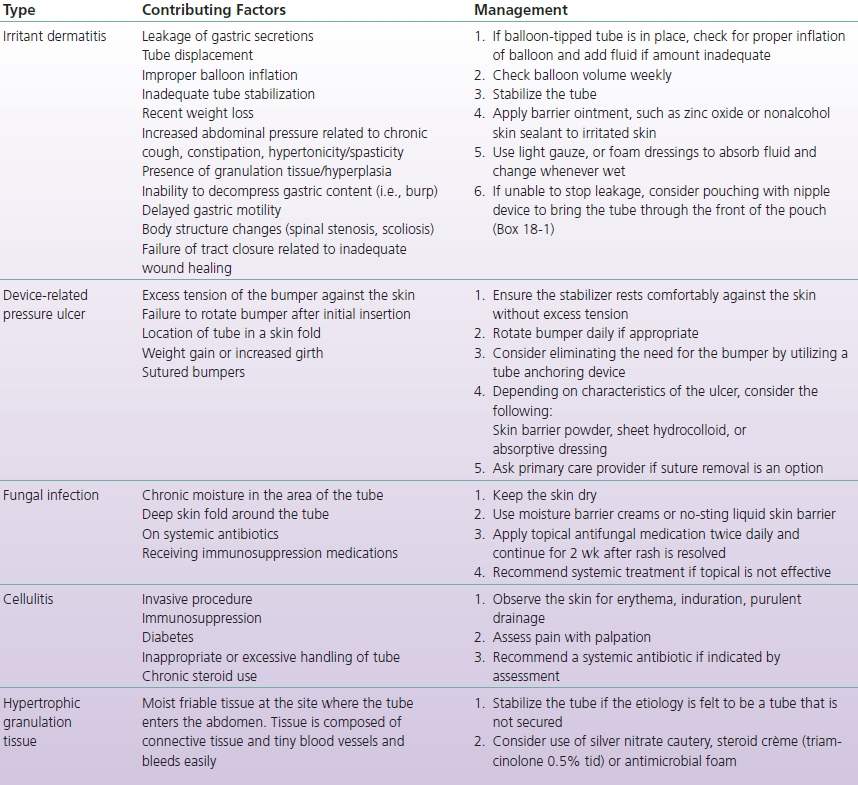
FIGURE 18-4. Drain tube attachment device.
Hypertrophic Granulation
It is thought that a poorly secured tube or one that migrates easily in and out of the skin opening may be a causative factor in the development of hypergranulation tissue around an enteral tube. Leakage of fluid, use of hydrogen peroxide, and poor fitting low-profile GT may also contribute to this overgrowth of tissue. The tissue itself is moist and often is friable, which contributes to leakage of formula and enteral fluid round the tube creating a cycle of leakage being both cause and effect. In some cases, it is painful to touch and may bleed easily. The presence of this tissue is not considered a serious complication, but there are reports in the literature linking it to wound infection and cellulitis around the tube (Rahnemai-Azar et al., 2014). A wide variety of treatment options from the application of topical antimicrobial agents and steroid creams to cauterization with silver nitrate and surgical removal have been described in the literature, but the evidence is anecdotal. Nurses in one community health district in the United Kingdom described a care routine for those persons (n = 25) in homes or care facilities with GTs and an overgrowth of granulation tissue around them. They used an antimicrobial cleanser and an antimicrobial foam dressing around the tube for 6 weeks and checked on them at 2-week intervals. At the end of the first 2 weeks, one third (n = 8) of the patients no longer had hypergranular tissue present. At the end of 6 weeks, the problem was resolved in six additional patients. The remaining patients received a silver alginate under a foam dressing, and if that did not resolve the hypergranulation, a steroid cream was applied (Warriner & Spruce, 2014). When hypergranulation tissue is present, it is important to stabilize the tube to reduce movement of the tube in the tract. In addition, clinicians use silver nitrate cautery, steroid cream (triamcinolone 0.5% applied tid), or an antimicrobial such as silver in or with a thin foam dressing. Silver nitrate sticks should be used with care to avoid getting the silver nitrate on intact skin as this may cause a burning sensation. More than one application may be needed. In extreme cases, surgical excision of the tissue may be required (WOCN, 2008).
Tube Replacement
GTs may become accidentally dislodged for a variety of reasons. The stabilizer may have loosened, water may have leaked from the balloon, inadvertent traction is placed on the tube, or the patient may have pulled it out. The latter cause is usually secondary to an altered mental state. In these patients, a low-profile tube may be appropriate (Fig. 18-5). The low-profile tube may also be used as a replacement tube when the patient wishes to have one for convenience and ease of concealing the tube under clothing. It is required to measure the stoma tract; there are measuring devices that determine the size tract for low-profile tube needed. This is especially important as a child is growing and may need to order another size tube. GT replacement may be done by a nurse, but verification of workplace policies and regulations of the state board of nursing should guide the decision to do this. In a healthy person, the tract in which the GT is placed would be healed in 2 to 3 weeks. The patients requiring enteral access for feeding are usually malnourished and have chronic conditions that may interfere with healing, so it is advisable to wait 4 to 6 weeks before a nurse should attempt tube replacement (McGinnis, 2013). There is a risk of inserting the tube in the peritoneum if the tract is not healed. When a tube is dislodged unexpectedly after 6 weeks from original placement, it must be replaced as soon as possible before the tract and the opening in the skin begins to close (Box 18-2). For patients at home, a family member may be taught to do this to avoid loss of access. Placing a tube into the opening will solve the immediate problem of maintaining the tract, but the tube should not be used until proper placement has been ascertained by return of gastric fluid through the tube (Juern & Verhaalen, 2014). Replacement GTs are preferred, but a Foley catheter may be used if a GT is not available. The Foley catheter is more readily available and is a less expensive option, but the lumen of the catheter will be smaller and it is less durable, so replacement with a GT should be done when one is available (Ojo, 2013).
CLINICAL PEARL
The patient should understand that when the tube falls out they should replace the tube immediately or seek medical attention for replacement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Nephrostomy Tubes
Nephrostomy Tubes Biliary Tubes
Biliary Tubes Conclusions
Conclusions