Chapter 10. Narcotic analgesics
Summary140
Analgesics in patients with terminal disease141
Determination of the cause of pain 141
The concept of total pain 141
Adjusting the dose of analgesic to keep the patient pain-free 142
Mild pain 142
Moderate-to-severe pain 142
Side-effects 143
Other routes of administration 143
Opioid non-responsive pain 143
Other methods 143
Analgesics in non-painful terminal disease 143
Analgesics and chronic non-terminal pain144
At the end of this chapter, the reader should be able to:
• outline the gating theory of pain
• describe what opium is, the important opioid drugs, their therapeutic uses and adverse effects
• describe the symptoms of morphine and heroin overdosage and emergency treatment
• describe what is meant by an opioid agonist, partial agonist and antagonist and give examples of each
• discuss the concept of ‘total pain’
• discuss the need for individualized pain control programmes
• explain the need to determine the cause of pain in patients with terminal disease
• describe the treatments for opioid non-responsive pain
Analgesics are drugs that relieve pain. They are of great importance in patient care, as pain is a common and distressing feature of many conditions. It must be remembered, however, that pain has its uses, both as a warning of the presence of disease and also, by its nature, it may help in localization and diagnosis of the underlying cause. It is debatable whether chronic pain has any physiological role, except as an unwelcome and constant reminder of ongoing tissue damage.
The perception of pain
The most important principle underlying pain perception is that the brain perceives pain. No matter how bad the damage to the body is, if nerve connections carrying pain signals to the brain are interrupted – for example, through damage to the spinal cord – the patient will feel no pain.
Mechanism of pain perception
The gating theory of pain
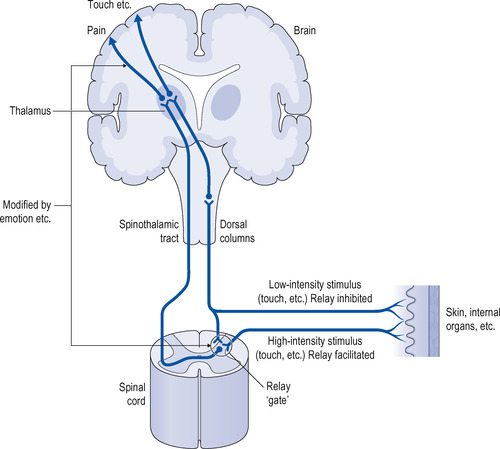
The mechanism of pain perception is summarized in Figure 10.1. The central nervous system (CNS) is constantly receiving nerve impulses arising in the body from the skin and internal organs. Under certain circumstances the brain interprets these as pain. There are a number of theories to explain how this occurs and the most popular today is the ‘gate’ (input control) theory. This states that:
• high-intensity stimulation activates a network of fine nerves at the periphery that terminate centrally in the posterior horn of the spinal cord
• from here, nerve impulses are relayed via the spinothalamic tract to the thalamus in the brain, where they are felt as pain
• there is then a further relay system to the cerebral cortex, where discrimination and interpretation occur.
 |
| Figure 10.1 Pathways involved in perception of pain. |
The passage of nerve impulses through the relay ‘gate’ in the posterior horn is modified by:
• ascending impulses from the periphery to the brain
• descending impulses from the brain to the periphery.
Ascending impulses from the periphery to the brain
Low-intensity ascending nerve impulses unrelated to pain dampen the transmission of pain impulses. This explains why methods such as transcutaneous stimulation or counter-irritation applied to a painful area can relieve pain by ‘closing the gate’. An example is the application of creams containing skin irritants to painful joints or muscles (e.g. Deep Heat cream, which contains capsicum extracted from hot chillies). Heat therapy, for example, through the use of lamps and heated pads, is also used to treat pain.
High-intensity impulses from an area of tissue damage (as may occur after surgery) overcome the low-intensity ascending nerve impulses and facilitate transmission through the ‘gate’ and thus increase painful sensations. This may be reduced by blocking impulses from the area of damage by local anaesthesia or by giving an analgesic just before an operation.
Drugs that relieve pain
Sites of drug action
Drugs that relieve pain may act at various sites along the pain pathways:
• They may act on the brain and spinal cord and reduce the appreciation of pain. This is the major site of action of opioid analgesics.
• They may suppress conduction in nerves carrying impulses from the painful area. This is where local anaesthetics act.
• They may reduce inflammation and other causes of pain in the painful area. This is the site of action, for example, of the non-steroidal anti-inflammatory drugs (NSAIDs; see Chapter 11).
Analgesics can be broadly classified for practical purposes as:
• opioid analgesics (Table 10.1)
| Type | Name |
|---|---|
| Natural | Morphine; codeine |
| Synthetic | Diamorphine; methadone; pethidine; phenazocine; dextromoramide; dipipanone; dihydrocodeine; fentanyl |
• non-opioid analgesics.
Non-opioid analgesics and local anaesthetics are dealt with in later chapters.
Opioid analgesics
The term opioid is applied to any substance which has an opium-like action. The opioids are also called narcotics or narcotic analgesics, because of their well-known soporific effects.
Nearly all the opioids are potentially drugs of dependence and this subject is discussed in more detail on p. 29.
The mechanism of action of opioid analgesics and antagonists of opioid action
The body contains chemicals called endorphins and encephalins. These are the body’s own type of opioid. Two of these, β endorphin and metencephalin, act on special opioid receptors in the nervous system, particularly in the midbrain and posterior horn of the spinal cord. When these receptors are stimulated, transmission of nerve impulses related to pain are inhibited and the appreciation of pain is suppressed. It seems likely that β endorphin and metencephalin are part of a system in the brain that controls pain appreciation and may be involved in such phenomena as acupuncture. Opioid drugs also react with these receptors and thus relieve pain. There are several types of opioid receptor in the nervous system, but the most important for pain control by opioids are called μ receptors and are responsible for the analgesia, euphoria and respiratory depression seen with most opioid analgesics. Although the most important actions of the opioids occur in the CNS, there is now some evidence that they may also react with receptors on the peripheral nerves, which augments their analgesic action. Endorphins, incidentally, are released during physical exercise and may be responsible for the feeling of well-being that participation in sports so often engenders.
Opioids and related drugs
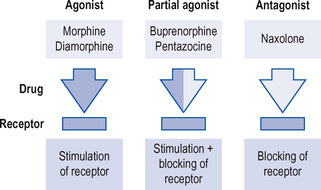
Some opioids, for example morphine and diamorphine (heroin), are full agonists. Some, such as buprenorphine, are partial agonists. Naloxoneis a drug that is a pure antagonist at opioid receptors (see Fig. 10.2).
 |
| Figure 10.2 Mode of action of opioid agonists, antagonists and partial agonists. |
Agonists
Examples are:
• morphine
• diamorphine
• methadone
• pethidine (meperidine)
• codeine
• dihydrocodeine
• dextropropoxyphene.
Partial agonists
Examples are:
• buprenorphine
• meptazinol
• nalbuphine
• tramadol.
Antagonists
Examples are:
• naloxone
• naltrexone.
Opium – the source of morphine
Opium is obtained from the unripe seed capsule of the poppy Papaver somniferum. Opium has been used for thousands of years in the Orient, and was (and possibly still is) smoked in opium dens. The drug produces euphoria and the user slips into a deep sleep, called the ‘yen’, which is often characterized by vivid dreams. The poppy is now grown in the UK as well (at secret locations) for commercial development of morphine. Crude opium is a brownish gum-like material and contains a number of substances, the most important being morphine, codeine and papaverine. Morphine is the most powerful of these alkaloids and the actions of morphine and opium are similar and may be considered together.
Opioid agonists
Morphine
Administration of morphine:
Oral
Morphine can be given orally as an immediate-release tablet that must be given every 4 hours. For long-term control of pain there are slow-release tablets, which are only needed twice daily, or as an aqueous solution.
When given orally as a single dose, morphine’s effect is greatly reduced because the liver breaks down about 75% of the dose through first pass metabolism before the drug reaches the circulation. With repeated oral dosage, however, it is very effective. This may happen because the metabolite morphine-6-glucuronide (see below) is slowly excreted and with repeated doses accumulates sufficiently to help to produce satisfactory analgesia.
Injection
The modes of injection are:
• subcutaneous
• intravenous
• continuous subcutaneous infusion.
When given by injection, morphine produces analgesia rapidly. The analgesic effect of morphine usually lasts about 4 hours after injection but depends to some extent on the severity of the pain, the sensitivity of the patient to the drug, and on the dose. The dosage in severe pain or in acute left ventricular failure depends on many circumstances, including the age, weight and general health of the patient.
Morphine can also be given slowly intravenously. The analgesic effect starts within 20 minutes of subcutaneous injection and 10 minutes of intravenous injection, and peaks after about 1 hour.
Morphine in small doses can also be given by continuous subcutaneous infusion. This allows the dose to be modified as required and can be very useful in severe and fluctuating pain. However, this method needs careful titration of the dose in relation to the therapeutic effect and fixed dose regimens are not very successful.
Metabolism and excretion
After absorption, morphine is combined in the liver to form several substances, one of which (morphine-6-glucuronide) has powerful analgesic properties of its own. The kidney excretes these substances. Repeated doses of morphine will induce a state of tolerance to the drug, so that increasing doses may be required to produce an effect.
CNS actions
The most important actions of morphine are on the CNS: the effects may be divided into depressant and stimulant. Morphine also causes the development of tolerance and dependence to its central actions. Drug dependence is discussed more fully in Chapter 22.
CNS depressant effects
• Morphine depresses the appreciation of pain by the brain and thus acts as a powerful analgesic.
• It relieves all types of pain.
• If the pain is felt at all, it seems to have lost its unpleasant nature.
• Morphine depresses the emotional component of pain, namely anticipation and fear of pain. It is euphoric and allays anxiety.
• It depresses respiration in large doses; patients are often left in pain through erroneous fears about respiratory depression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
