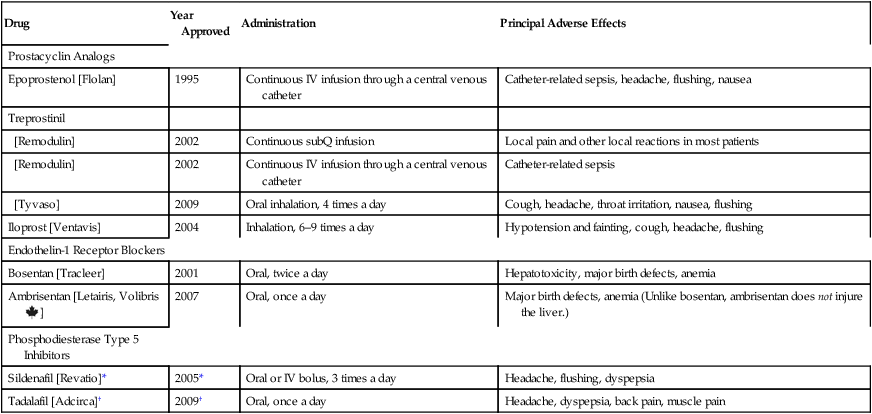
CHAPTER 107 Since 1995, seven new drugs for PAH have been approved: epoprostenol, treprostinil, iloprost, bosentan, ambrisentan, sildenafil, and tadalafil (Table 107–1). All seven promote pulmonary vasodilation. In addition, they may delay or reverse pulmonary vascular remodeling. Also, all seven are very expensive. Discussion below is limited to these seven drugs. TABLE 107–1 Drugs for Pulmonary Arterial Hypertension *Sildenafil is also sold as Viagra for treating erectile dysfunction (ED). Approval for ED was granted in 1998. †Tadalafil is also sold as Cialis for treating ED. Approval for ED was granted in 2003. The phosphodiesterase type 5 (PDE5) inhibitors reduce pulmonary arterial pressure by causing dilation of pulmonary blood vessels. Two PDE5 inhibitors—sildenafil and tadalafil—are approved for PAH. Both drugs were originally developed for erectile dysfunction (ED). The basic pharmacology of these drugs and their use for ED are discussed in Chapter 66 (Drugs for Erectile Dysfunction and Benign Prostatic Hyperplasia). Consideration here is limited to their use in PAH. When preterm delivery cannot be prevented, injecting the mother with glucocorticoids can accelerate fetal lung maturation, and can thereby decrease the incidence and severity of RDS. Glucocorticoids act by stimulating production of fibroblast pneumocyte factor, which in turn stimulates production of surfactant by fetal pneumocytes. Glucocorticoids are effective when used during weeks 24 to 34 of gestation. Beyond week 34, fetal lungs are sufficiently mature that no benefit is gained by giving these drugs. Two drugs are recommended: dexamethasone and betamethasone. A single course consists of either (1) dexamethasone, 6 mg IM every 12 hours for four doses, or (2) betamethasone, two 12-mg IM doses, injected 24 hours apart. To be effective, the last glucocorticoid dose should be administered at least 24 hours before delivery, but no more than 7 days before. The basic pharmacology of the glucocorticoids is discussed in Chapters 60 and 72. These enzymes are given as replacement therapy. All preparations contain lipase, protease, and amylase. The most effective formulations deliver the enzymes in enteric-coated microspheres, which are designed to protect the enzymes from stomach acid and ensure dissolution in the duodenum, the site where the enzymes act. Pancreatic enzymes are discussed in Chapter 80. With tobramycin [TOBI], the dosage is 300 mg every 12 hours in repeating cycles of 28 days on and 28 days off. Each dose takes 10 to 15 minutes to administer. Common adverse effects include cough, wheezing, and hoarseness. In contrast to IV aminoglycosides, inhaled tobramycin is unlikely to cause hearing loss, although it can cause tinnitus (ringing in the ears). The basic pharmacology of tobramycin and other aminoglycosides is discussed in Chapter 87. With aztreonam [Cayston], the dosage is 75 mg 3 times a day in repeating cycles of 28 days on and 28 days off. Each dose takes 2 to 3 minutes to administer, making aztreonam more convenient than tobramycin. Principal adverse effects are cough, nasal congestion, and wheezing. The basic pharmacology of aztreonam is presented in Chapter 85. For patients undergoing an acute crisis, analgesics and hydration are the cornerstone of treatment. Unfortunately, most patients generally fail to receive adequate pain relief. If the pain is moderate, a nonopioid analgesic (acetaminophen or a nonsteroidal anti-inflammatory agent) may be sufficient. However, if pain is severe, intensive therapy with an opioid is required. Parenteral (IV or IM) morphine and meperidine have been employed. Patient-controlled analgesia may be especially effective. The basic pharmacology of the opioids is discussed in Chapter 28. High doses of intravenous methylprednisolone (a glucocorticoid) can shorten the duration of a sickle cell crisis. The drug should be used together with, not instead of, an opioid. Unfortunately, when glucocorticoids are discontinued, rebound pain may occur. The basic pharmacology of the glucocorticoids is discussed in Chapters 60 and 72.
Miscellaneous noteworthy drugs
Drugs for pulmonary arterial hypertension

Drug
Year Approved
Administration
Principal Adverse Effects
Prostacyclin Analogs
Epoprostenol [Flolan]
1995
Continuous IV infusion through a central venous catheter
Catheter-related sepsis, headache, flushing, nausea
Treprostinil
[Remodulin]
2002
Continuous subQ infusion
Local pain and other local reactions in most patients
[Remodulin]
2002
Continuous IV infusion through a central venous catheter
Catheter-related sepsis
[Tyvaso]
2009
Oral inhalation, 4 times a day
Cough, headache, throat irritation, nausea, flushing
Iloprost [Ventavis]
2004
Inhalation, 6–9 times a day
Hypotension and fainting, cough, headache, flushing
Endothelin-1 Receptor Blockers
Bosentan [Tracleer]
2001
Oral, twice a day
Hepatotoxicity, major birth defects, anemia
Ambrisentan [Letairis, Volibris ![]() ]
]
2007
Oral, once a day
Major birth defects, anemia (Unlike bosentan, ambrisentan does not injure the liver.)
Phosphodiesterase Type 5 Inhibitors
Sildenafil [Revatio]*
2005*
Oral or IV bolus, 3 times a day
Headache, flushing, dyspepsia
Tadalafil [Adcirca]†
2009†
Oral, once a day
Headache, dyspepsia, back pain, muscle pain
Phosphodiesterase type 5 inhibitors
Drugs for neonatal respiratory distress syndrome
Prenatal and postnatal glucocorticoids
Drugs for cystic fibrosis
Drug therapy
Nutritional drugs
Pancreatic enzymes.
Pulmonary drugs
Inhaled antibiotics for chronic suppressive therapy.
Drugs for sickle cell anemia
Analgesics and glucocorticoids
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Miscellaneous noteworthy drugs
Only gold members can continue reading. Log In or Register to continue
