Introduction
‘A practising midwife shall only supply and administer those medicines, including analgesics, in respect of which she has received the appropriate training as to use, dosage and methods of administration’ (Rule 7: Administration of medicines, Nursing and Midwifery Council (NMC), 2004).
This chapter discusses the midwife’s position regarding the supply and administration of medicines. It includes a pharmacopoeia listing some of the commonest drugs used during the intrapartum period. While every care has been taken to ensure this information is correct and up to date at the time of publication, it is intended as a guide only and midwives are advised to confirm information by referring to the NMC, local protocols and the British National Formulary (BNF) or MIMS which are updated as new evidence emerges.
Facts
- Most medicines the midwife supplies or administers are covered under midwife exemptions.
- Midwifery practice is subject to medicines legislation: the Medicines Act 1968, the Misuse of Drugs Act 1971, the Midwives Rules and Standards (NMC, 2004) and the NMC (2007) Standards for Medicines Management.
- Midwives should expect their supervisor of Midwives (SOM) to audit their drug administration records periodically (NMC, 2004).
- A woman has the right to use homeopathic and herbal medicines. However, if the midwife believes that using the medicines might be counterproductive she should discuss this with the woman (NMC, 2004).
- Midwives practising outside their employing authority, or outside the NHS, should seek advice from their SOM regarding any matters related to the supply, administration, storage, surrender and destruction of controlled drugs and other medicines (NMC, 2004).
- A woman who has not used a controlled drug, which has been prescribed by her general practitioner, can return the drug to the pharmacist from where it was obtained. Midwives must not do this for her. Alternatively, the woman can choose to destroy it – ideally with the midwife as a witness (NMC, 2004).
Drugs are classified as:
- General sales list (GSL): obtainable from retail outlets, e.g. supermarkets.
- Pharmacy (P): no prescription required but can only be bought from a pharmacy.
- Prescription only medicines (POM): may only be sold or supplied with a prescription.
Midwives and the supply and administration of medicines
Midwives exemptions
Provided it is in the course of their practice, registered midwives can supply and administer, on their own initiative, any medicines specified under midwives exemptions.
Under these exemptions midwives may supply/administer:
- Without the need for a prescription or patient-specific written direction from a medical practitioner.
- Without the need for a patient group direction (PGD).
All ‘General sales list’ medicines
All ‘Pharmacy’ medicines
- Examples of P and GSL medicines that midwives commonly use in their practice are paracetamol (GSL/P), oral iron preparations (P), oral laxatives (GSL/P) and entonox (P) (Royal College of Midwives (RCM), 2006).
Certain ‘Prescription Only medicines’ are also covered
- Midwives can supply/administer specific POM in the course of their practice. These POMs are listed by the NMC (2004) and the RCM (2006).
- Examples of POMs the midwife can administer under the exemptions include diamorphine (heroin), lignocaine, oxytocins, pethidine hydrochloride and phytomenadione (vitamin K).
- Intravenous fluids are not covered under midwives exemptions.
- Any medicine covered under the midwife exemptions that appears in the pharmacopoeia below has been highlighted with*.
Standing orders
Many midwives work in units that provide standing orders. Standing orders may be helpful simply because they give written guidance on the route of administration, dosage and specific circumstances where midwives may supply and administer medicines.
However, standing orders are not required in law under the current legislation and the actual term ‘standing order’ does not exist in any medicines legislation (NMC, 2005).
The NMC states that there is no legal requirement to replace standing orders with patient group directions if the administration and supply of medicines on standing orders is covered under the midwives exemptions.
Patient group directions
Patient group directions (PGDs) provide a local framework for the supply and administration of medicines by certain health professionals without a prescription. These medicines are approved for supply/administration by local doctors and pharmacists for patients in pre-identified clinical situations.
This is a complex topic and there is much confusion about whether midwives should be involved in PGDs. Part of the confusion is due to the fact that PGDs are intended for all health professionals, not just midwives.
- Most POMs the midwife uses are covered under midwives exemptions. If this is not the case, the midwife will require either a prescription or a PGD.
- None of the midwives exemptions has been replaced by the legislation concerning PGDs and there is, therefore, no legal requirement to move all existing locally agreed policies into PGDs.
- PGDs are not a form of prescribing, and while midwives should ideally be involved in drawing up and signing off PGDs, a PGD must be signed off by a doctor and pharmacist involved in the PGD development (NMC, 2007).
- PGDs can only be administered by midwives named in each PGD document: PGD administration cannot be delegated.
See also PGD guidance under ‘Useful contacts’.
Documentation, record keeping and drug errors
Midwives are required to keep accurate, detailed records of the supply/administration of all medicines. Below are the guidelines for best practice adapted from the NMC.
Safety and good practice
- Correctly identify the woman or baby to whom the medicine is to be administered.
- Check that the woman is not allergic to the medicine before administration.
- Consider the dosage, method of administration, route and timing in the context of the woman’s condition and any co-existing therapies.
- Check that the prescription, or the label on the medicine dispensed by a pharmacist, is clearly written and unambiguous.
- Check the expiry date.
Documentation
- Document immediately all medicines administered; avoid abbreviations.
- Ensure that all written entries are clear and legible, accurately dated, timed and signed with the signature printed alongside the first entry.
- Clearly countersign the signature of any student who is being supervised in the administration of medicines.
Errors in drug administration
- If a midwife makes or identifies an error in the administration of a drug, it should be reported immediately to the prescriber and the line manager/employer. A practising midwife should also inform their named SOM (RCM, 2006; NMC, 2007). Any error or near miss should be reported to the local risk management team.
To avoid errors in drug administration the NMC recommends that:
- A second registered professional checks any complex drug calculation.
- It is unacceptable to prepare substances for injection in advance of their immediate use. A practitioner does not administer medication drawn up by another practitioner when not in their presence (NMC, 2007).
The NMC supports the use of critical incident procedures for clinical errors but urges managers, when considering disciplinary action in relation to the administration of medicines to distinguish between:
‘those cases where the error was the result of reckless or incompetent practice or was concealed, and those that resulted from other causes, such as serious pressure of work, and where there was immediate, honest disclosure in the patient’s interest’ (NMC, 2007).
Common abbreviations
The Joint Formulary Committee (2007) states that prescriptions should be in English without abbreviation; however, they do acknowledge that some abbreviations are used. Because of this, midwives should be able to use or interpret the abbreviations and their meaning (see Tables 24.1–24.3).
Table 24.1 Route of administration.
| Abbreviation | Route of administration |
| IM | Intramuscular |
| IV | Intravenous |
| sc | Subcutaneous |
| po | per oram Oral |
| pr | per rectum Rectal |
| pv | per vaginum Vaginal |
Drug calculations
Formula for liquids:
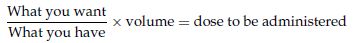
100 mg of penicillin is prescribed; it comes as a preparation of 125 mg of penicillin in 5 ml solution
Example:
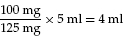
Table 24.2 Frequency of drug administration.
| Abbreviation | Frequency |
| stat | Immediately |
| od omnl die | Once a day |
| bd bis die | Twice a day |
| tds ter die sumendus | Three times a day |
| qds quatre die sumendus | Four times a day |
| nocte | At night |
| prn pro re nata | As needed |
| hrly | Hourly |
Table 24.3 Units of measurement.
| μg or mcg | Microgram |
| ng | Manogram |
| mg | Milligram |
| g | Gram |
| kg | Kilogram |
| ml or mL | Millilitre |
| l or L | Litre |
| IU | International units |
| mU | Milliunits |
Pharmacopoeia of intrapartum drugs
Listed in alphabetical order below are some of the commonest drugs used in the intrapartum period. Drugs used in resuscitation are not listed here; please refer to Chapter 17 for more information.
Substances covered under midwife exemptions that appear in the list below have been highlighted with *.
IV fluids are not covered under midwives exemptions, so for example if it is planned to administer any drugs covered by midwife exemptions (e.g. syntocinon) in IV fluids, a prescription or PGD would be required.
Drug: Atosiban (Tractocile®) (POM)
| Action: | Myometrial relaxant. |
| Dosage and frequency: | IV injection: |
| |
| |
| Maximum treatment duration 48 hours. | |
| Indications: | To delay uncomplicated preterm delivery 24 – 33 weeks gestation: allowing administration of corticosteroid therapy or transfer to specialist unit, following which is no benefit in continuing the infusion (Royal College Obstetricians and Gynaecologists (RCOG), 2002). |
| Route: | IV bolus/infusion. |
| Contraindications: | Severe pre-eclampsia or eclampsia, active bleeding/placenta praevia (as betamimetics relax the uterus); fetal death, preterm rupture of membranes >30 weeks and/or infection (where there is probably no benefit to stop labour) (Keirse, 2000; RCOG, 2002). |
| Side effects: | Tachycardia, nausea, vomiting, hypotension, headache, dizziness, hot flushes, hyperglycaemia, less commonly rash, fever, pruritus. |
| Cautions: | Intrauterine growth restriction; hepatic and renal impairment. Monitor postnatal blood loss. |
| Note: | RCOG (2002) suggests ‘it is reasonable not to use tocolytic drugs, as there is no clear evidence that they improve perinatal outcome’ although as stated they may gain time if necessary, e.g. intrapartum transfer to a specialist centre. Tocolytic drugs have serious side effects; nifedipine (not licenced for this use in the UK) or atosiban is more effective and has fewer adverse effects than ritodrine (RCOG, 2002). |
Drug: Betamethasone/dexamethasone (POM)
| Action: | Anti-inflammatory. |
| Dosage and frequency: | • 12 mg first dose. |
| • 12 mg second dose 12 hours later. | |
| Indications: | Prophylactic treatment administered to the mother at risk of preterm birth to promote surfactant production encouraging fetal lung maturation. |
| Route: | Oral, IM. |
| Contraindications: | Systemic infection (unless responding to appropriate treatment). Refer to JFC (2008). |
| Side effects: | Side effects from steriods can be numerous, although these are often associated with longer-term steroid use and are listed extensively in the British National Formulary of drugs (BNF) (JFC, 2008). Repeated prenatal exposure may suppress adrenal function in the baby but is rarely clinically significant (JFC, 2008); ongoing research is exploring possible more serious endocrine defects. |
| Cautions: | Diabetes in the mother. |
| Note: | See BNF for other uses of corticosteroids. Their use described here relates solely to prophylactic administration in threatened preterm labour. Every effort should be made to administer a prophylactic corticosteroid regime when clinical features indicate a possible preterm delivery (Confidential Enquiry into Stillbirths and Deaths in Infancy (CESDI), 2003; Roberts & Dalziel, 2007). |
Drug: Bupivacaine/ropivacaine hydrochloride (Naropin R®) (POM)
| Action: | Local anaesthetic. |
| Dosage and frequency: | Adjusted by the anaesthetist according to the woman’s physical status and weight. |
| Initial labour lumbar block: | |
| • Bupivacaine: 3–6 ml depending on concentration, | |
| • Ropivacaine: 10–20 ml 2 mg/ml solution. Continuous epidural infusion in labour (once block established): | |
| • Bupivacaine: 10–15 mg/h of 0.1% (1 mg/ml) or 0.125% (1.25 mg/ml) solution. | |
| • Ropivacaine: 6–14 ml/h of 0.2% (2 mg/ml) solution. | |
| Indications: | Regional local anaesthetic for labour pain, caesarean section, post-delivery procedures (e.g. suturing, manual removal of placenta). |
| Route: | Lumbar epidural/spinal. |
| Contraindications: | Hypovolaemia, hypotension, maternal infection, coagulation disorder or ongoing coagulation treatment, cardiac/respiratory impairment, epilepsy, complete heart block (JFC, 2008). |
| Side effects: | Central nervous system effects: respiratory depression, convulsions, hypotension and bradycardia, also pyrexia and leg weakness. Ropivacaine may reduce risk of cardiac symptoms. |
| Note: | Epidural/spinal anaesthesia can have secondary effects, i.e. poor mobility and less upright position leading to prolonged labour, increased fetal malposition, increased oxytocin augmentation and perineal trauma due to increased instrumental delivery (Leighton & Halpern, 2002; Lieberman & O’Donaghue, 2002; Howell, 2004). There may also be more subtle effects on natural mothering behaviour: maternal endorphin levels appear lower with epidurals (Abboud et al., 1983). |
Drug: Codeine, dihydrocodeine and paracetamol preparations* (including (POM/P) Co-codamol®, Co-dydramol®)
| Action: | Analgesic. |
| Dosage and frequency: | 1–2 tablets 4–6 hourly; maximum dose 8 tablets in 24 h. |
| Indications: |



