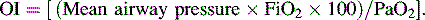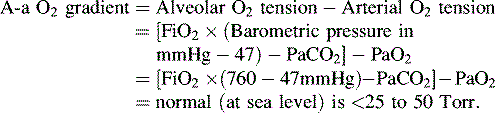7 Mechanical Support of Cardiopulmonary Function
Extracorporeal Membrane Oxygenation, Ventricular Assist Devices, and the Intraaortic Balloon Pump
Pearls
• Signs of critically low cardiac output requiring mechanical circulatory support include: mixed venous oxygen saturation less than 40%, increased ventricular end-diastolic filling pressure, cardiac index less than 2 L/min per m2 body surface area (BSA), persistent metabolic acidosis, oliguria, poor peripheral perfusion, increased inspired oxygen requirements, and poor ventricular function by echocardiogram.
• Mechanical circulatory support (MCS) is indicated when low cardiac output persists despite maximal medical treatment, including afterload reduction combined with inotropes/catecholamines, diuretics, a phosphodiesterase-III inhibitor, fluid and transfusion management, and mechanical ventilation.
• In the pediatric population, ventricular assist devices (VADs) are used to support the failing heart as a “bridge to recovery” or “bridge to transplant.”
• MCS should be anticipated and every attempt should be made to initiate support before the development of end-organ dysfunction or circulatory collapse.
• Persistent low filling of a left ventricular assist device (LVAD) pump with rising right atrial pressure is an indication of right-sided heart failure. An echocardiogram is indicated to evaluate ventricular function and check for a pericardial effusion.
• The oxygen index (OI) is often used to identify possible indication for extracorporeal membrane oxygenation (ECMO) for children with respiratory failure.
• Alveolar-arterial (A-a) gradient of 600 to 624 mm Hg is a clinical indication for ECMO. This is computed as follows (NOTE: 47 mm Hg = partial pressure of water vapor):
• Signs to change venovenous (VV) ECMO to venoarterial (VA) ECMO include: persistent hypoxia in the presence of hypotension and poor cardiac output on maximal ventilator and inotropic support, nonperfusing arrhythmias, or cardiac arrest.
Introduction
The child with life-threatening cardiac or respiratory failure that is refractory to maximal medical support may require MCS. Over the last few years, substantial advances in pediatric MCS have occurred, with expanding indications for use, greater availability of devices suitable for pediatrics, and improved outcomes.16,20
ECMO and centrifugal ventricular assist devices remain the mainstay of MCS for children.23 MCS is not a treatment but rather a therapeutic support to provide adequate tissue oxygen delivery and maintain end-organ perfusion and function while the reversible disease responds to treatment. This support has many potential complications and should be used in appropriate patients with specific criteria for initiation.
Use of MCS in the pediatric population requires knowledge of the pathophysiology and treatment of cardiac and respiratory failure (see Chapters 6 to 9). In addition, the critical care nurse and the ECMO specialist require advanced training in MCS, because they must be familiar with the function of the support device, components of the circuit or console, potential complications, and troubleshooting for the device.
Device Selection
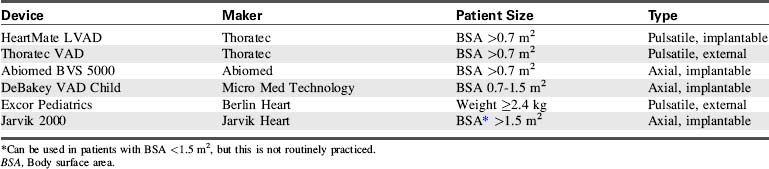
The selection of MCS device is influenced by the indication for the device, the duration of support anticipated, and the devices available. Devices are categorized for short- or long-term support. ECMO, centrifugal VADs, and intraaortic balloon pumps (IABPs) are considered short-term modalities. Pulsatile and rotary-axial devices are typically considered long-term modalities.11
Pediatric MCS offers a potential “bridge to transplant” or a “bridge to recovery.” The term destination therapy is used to describe long-term support for patients who are not heart transplant candidates and those whose heart function is unlikely to recover. Currently, no devices are approved by the U.S. Food and Drug Administration (FDA) for destination therapy in children. Three devices approved by the FDA for destination therapy in adults are the HeartMate VE/XVE LVAS and HeartMate II (Thoratec Corporation, Pleasanton, Calif.), and the AbioCor Implantable replacement heart (Abiomed, Danvers, Mass.).11,55
Extracorporeal membrane oxygenation
ECMO remains the most common form of MCS in the pediatric population. ECMO support evolved directly from cardiopulmonary bypass devices beginning with development of the membrane oxygenator in the 1950s.13,41 In the 1970s, intraoperative cardiopulmonary bypass was successfully used for infants during surgical correction of congenital heart disease.3 In 1972, successful ECMO support of a 24-year-old trauma victim with acute respiratory distress syndrome by Hill et al.37 led to a multicenter National Institutes of Health clinical trial of ECMO versus medical management of adult acute respiratory distress syndrome that failed to show a survival advantage with ECMO support, but did lead to widespread improvement in mechanical ventilation in adults.
In 1976, Bartlett et al.6 reported the first successful use of ECMO for treatment of severe neonatal respiratory distress. Since that time, ECMO has been used successfully in the treatment of neonates with meconium aspiration, congenital diaphragmatic hernia, pneumonia, sepsis, or persistent pulmonary hypertension.57 ECMO is now used as rescue therapy for pediatric patients with severe refractory respiratory or heart failure unresponsive to maximal conventional treatment.
In 1989, the ECMO centers formed a national organization, the Extracorporeal Life Support Organization to coordinate clinical research, develop ECMO guidelines, and maintain a national registry of all ECMO centers and cases. ECMO follow-up data have documented the cost effectiveness of ECMO compared with conventional support and improved patient outcomes with the use of ECMO.49
ECMO Terminology
The two types of ECMO support are VA and VV ECMO. VA ECMO provides both cardiac and respiratory support, whereas VV ECMO provides only pulmonary support. The differences between the VA and VV ECMO are summarized in Table 7-1.
Table 7-1 Differences Between VA and VV ECMO
| VA ECMO | VV ECMO |
| Achieves higher PaO2 | Achieves lower PaO2 |
| Requires lower perfusion rates | Requires higher perfusion rates |
| Bypasses pulmonary circulation | Maintains pulmonary blood flow |
| Decreases pulmonary artery pressures | Elevates mixed venous PO2 |
| Provides cardiac support to assist systemic circulation | Does not provide cardiac support to assist systemic circulation |
| Requires arterial cannulation | Requires only venous cannulation |
ECMO, Extracorporeal membrane oxygenation; PaO2, partial pressure of arterial oxygenation; PO2, partial pressure of oxygen; VA, venoarterial; VV, venovenous.
Venoarterial ECMO
Circuit
The VA ECMO circuit is composed of polyvinyl chloride tubing attached to venous and arterial cannulas (Fig. 7-1). The venous cannula is inserted into the internal jugular vein and advanced through the superior vena cava and right atrium to the tricuspid valve. The arterial cannula is placed into the right common carotid artery with the tip of the cannula advanced to the innominate artery. Postoperative cannulation immediately after cardiac surgery is often accomplished through the median sternotomy incision with direct cannulation of the right atrial appendage and the aorta. In adolescent or adult patients, the femoral artery and vein are often used for VA ECMO cannulation.
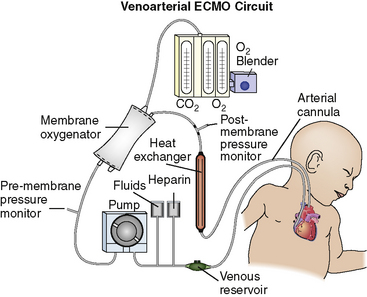
Fig. 7-1 Venoarterial extracorporeal membrane oxygenation circuit.
(From Biddle M, Gulanick M, Berra K: Interdisciplinary team in cardiac rehabilitation. In Moser DK, Riegel B, editors: Cardiac nursing: a companion to Braunwald’s heart disease. Philadelphia, 2008, Saunders.)
Hemodynamic Changes During Venoarterial ECMO
ECMO Support with Single Ventricle Physiology
In patients with single ventricle physiology or shunt-dependent pulmonary blood flow, controversy exists about management of the shunt during MCS. There is debate regarding whether the shunt should be completely closed, partially closed, or left open during MCS. The challenge is to maintain balance between systemic and pulmonary circulation. If the shunt is completely closed during support, there is a risk of pulmonary infarction. If the shunt is open during ECMO, increased ECMO flow may be needed to maintain adequate pulmonary and systemic blood flow and systemic oxygen delivery.53
There are limited data regarding the role of ECMO after stage 1 palliation (and its variants) for hypoplastic left heart syndrome. ECMO is a useful tool for treatment of potentially reversible conditions, such as acute shunt thrombosis and transient depressed ventricular function53; ECMO may be used electively in the immediate postoperative period. However, the use of ECMO after the Norwood procedure remains controversial because the infant’s anatomy is complicated, the therapy is expensive, and survival rate is low.18,51,53,57
Venovenous ECMO
Hemodynamic Changes during Venovenous ECMO
Although there is no difference in the rate of survival, or rates of intracranial hemorrhage and infarction, seizures and brain death in pediatric respiratory patients for VV versus VA ECMO, VV ECMO is associated with significantly more cannula problems, higher incidence of CPR on ECMO, and use of inotropic support. In addition, there is also a trend toward more renal failure and use of hemofiltration on VV ECMO.62
Indications and Contraindications of ECMO Support
The indications and contraindications differ for neonatal, pediatric, and cardiac ECMO. Although specific inclusion and exclusion criteria exist, the use of ECMO remains a center specific decision and has to be considered on a case-by-case basis. The inclusion criteria for neonatal and pediatric ECMO are summarized in Box 7-1.
Box 7-1 Inclusion Criteria for Neonatal and Pediatric Respiratory ECMO Support
Pediatric
• Potentially reversible etiology for pulmonary failure
• Oxygenation index >40 and worsening respiratory failure despite maximal ventilator support
• Ventilator support for <14 days
• No other major contraindication to ECMO support such as severe central nervous system abnormality, ongoing hemorrhagic condition or coagulopathy, or multiple organ system failure in >3 organ systems
Neonatal Respiratory Failure
Contraindications for neonatal ECMO include the presence of irreversible and uncontrolled bleeding or coagulopathy, IVH greater than grade II, birth weight <2 kg, or gestational age <34 weeks, lethal chromosomal abnormality or congenital malformation incompatible with life, and duration of mechanical ventilation >14 days.43
With more extensive use of therapies such as high-frequency ventilation, surfactant, and nitric oxide, there has been a marked decline in the need for neonatal ECMO.38 If patients do not respond to these therapies, ECMO should be offered in a timely manner.
Pediatric Respiratory Failure
There is less consensus regarding indications for ECMO support for pediatric patients than for neonates with respiratory failure. Severe respiratory failure in children has many etiologies. A child is generally considered to be a candidate for ECMO if death is believed to be nearly certain despite maximal conventional therapy, and the lung disease is believed to be reversible and other organ systems are intact.29 In children, the OI has not been shown to be as predictive of mortality as it is in neonates. Many centers use the criteria of OI >40 and rising, hypercarbia and pH <7.1, a PaO2:FiO2 ratio less than 100 and falling, ventilator support for less than 14 days, and no contraindications such as significant neurologic or hemorrhagic conditions, or failure of more than three organ systems.43 With the use of lung protective strategies and adjunctive therapies such as high-frequency ventilation, surfactant, and nitric oxide, it has become harder to establish criteria for initiation of ECMO support. Contraindications for pediatric ECMO are similar to those in the neonatal period; these include diagnoses incompatible with life, intractable hemorrhage or coagulopathy, or severe central nervous system abnormality.
Cardiac Failure
Cardiac ECMO support has steadily increased over the past decade.24 Although isolated left ventricular failure is relatively rare in children, right ventricular failure, pulmonary hypertension, and hypoxemia are often associated with circulatory collapse in children with congenital heart disease. The most common causes of circulatory failure in infants and children are cardiovascular surgery (postcardiotomy), end-stage cardiomyopathy, and acute myocarditis. The most common indications for ECMO in cardiac patients are severe hypoxia, severe pulmonary artery hypertension, cardiogenic shock, cardiac arrest, and failure to wean from cardiopulmonary bypass after surgical repair.
Contraindications for the use of cardiac ECMO include incurable malignancy, advanced and presumed irreversible multisystem organ failure, extreme prematurity, and severe central nervous system abnormality or hemorrhage.59 In addition, if a patient will not be a transplant candidate, the patient should be carefully evaluated before ECMO. During the past 10 years, many contraindications have been removed from the list or labeled as relative rather than absolute contraindications.
Weaning from ECMO
There are two basic approaches to weaning patients from VA ECMO. Neither method has been shown to be superior to the other.32
Weaning VV ECMO is slightly different than weaning VA ECMO. When weaning VV ECMO, after the ventilator settings are increased, both of the membrane oxygenator gas ports are isolated from ambient air. Eventually, the blood entering and exiting the membrane oxygenator is in equilibrium and reflects typical venous values. This eliminates the need to clamp the venous cannula and allows a longer trial off VV ECMO.32
ECMO Troubleshooting
ECMO Circuit Emergencies
• Is blood ejecting from the circuit?
• Is air being pumped in the circuit?
• Is the pump delivering forward flow?
• Does the post-oxygenator blood appear red?
• Is the sweep gas being delivered and vented from the oxygenator?
If there is no squirting blood or air being pumped, the patient can be maintained on ECMO while the problem is corrected. ECMO circuit emergencies and problem troubleshooting are summarized in Table 7-2.
Table 7-2 Troubleshooting the ECMO Circuit
| Problem | Signs and Symptoms | Response |
| Clots in circuit | Dark zones or streaks seen; can cause bleeding due to coagulopathy (decreased platelets or fibrinogen unresponsive to transfusion) | Monitor circuit, monitor for coagulopathy, change circuit |
| Oxygenator failure | Failure to remove CO2 or add adequate levels of oxygen in spite of increasing sweep gases and FiO2; may see blood or serum leaking from gas exhaust port | Remove air and debubble circuit if present; check that all gas lines are intact and not leaking to rule out gas line failure; replace oxygenator if it has failed |
| Air in venous side of circuit | Bubbles seen | Correct source of problem, remove air |
| Air in arterial side of circuit | Bubbles seen | Remove patient from ECMO by clamping arterial and venous lines to patient; place patient in Trendelenburg position; replace/repair the component identified as cause; remove all air, recirculate, and then return to ECMO |
| Power failure | Pump stops with no AC power | Plug into emergency hospital power; use battery or UPS; hand crank |
| Accidental decannulation | With partial removal on venous side, air entrainment can be seen; with complete decannulation, cannula will be out of the body with bleeding from cannulation site and possible pumping of air or blood | Cease ECMO and stop pump; put direct pressure on site; call surgeon for immediate replacement of cannula and to control bleeding; replace volume losses with available blood products and crystalloid |
| Water heater failure | Patient exhibits hypothermia; bradycardia with reflex hypertension may be seen with pallor | Obtain new water heater and connect to circuit |
| Raceway rupture | Blood spurts out of damaged tubing in pump head | Cease ECMO; turn pump off; replace raceway segment with new tubing; clean pump head; place new raceway segment into the pump head and recirculate; return to ECMO |
| Cracks in tubing or connectors or loose stopcocks | On venous side [negative pressure side] air will entrain into the circuit. On positive pressure side of the circuit, blood will leak out | Tighten connections or replace cracked tubing or connectors; cease ECMO; stop pump; cut out and replace the damaged segments |
AC, Alternating current; ECMO, extracorporeal membrane oxygenation; UPS, universal power source.
Ventricular assist devices
The options for MCS for infants and children with cardiac failure are limited. There are currently no devices approved by the FDA for specific use in infant patients. However, several devices have been used successfully to support infants and children through the following: off-label use of devices approved by the FDA for use in adults, compassionate use of investigational adult VADs, the use of devices that have received an FDA Humanitarian Device Exemption, the use of devices fabricated from FDA-approved components (such as ECMO circuits), and devices that are approved on a case-by-case basis by the FDA for emergency use.7,8,12,20–22,27,28,31,44,56 Table 7-3 lists ventricular assist devices currently being used in the pediatric population under various exemptions or adult approved devices used in older children.2,26,30,35,42,45,46,61 Additional information regarding VADs used in children is included in Evolve Table 7-1 in the Chapter 7 Supplement on the Evolve Website.
Components and Function of VAD Support
A VAD is a heart pump that can be used to support the right ventricle (called a right ventricular assist device [RVAD]), the left ventricle (called an LVAD), or both ventricles (called a BiVAD). Most VADs have three major components: a pump (located inside or outside the body), a control system, and an energy source. The control system and energy source are found outside the body. The energy source can be a battery or compressed air (pneumatic).23
In the pediatric population, most VAD pumps are extracorporeal (outside the body) and connected to inflow and outflow cannulae (Fig. 7-2). The critical care nurse should understand the components of the VAD and VAD function.
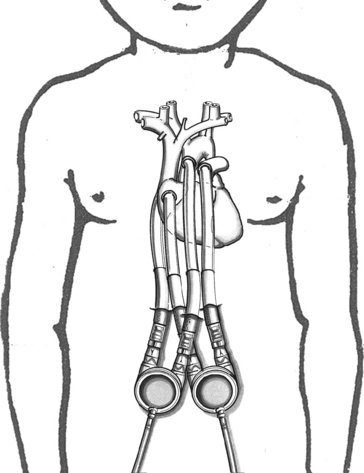
Fig. 7-2 Extracorporeal pneumatic biventricular assist device (Berlin Heart EXCOR).
(Redrawn from an illustration of the Berlin EXCOR by Berlin Heart, Berlin, Germany.)
The placement of the VAD cannula differs if the device is a bridge to recovery, compared with a bridge to transplantation. If the VAD support is serving as a bridge to recovery, the inflow cannula is often connected to the patient’s atrium.19 This cannulation is technically easier and spares the ventricle further injury. However, ventricular cannulation enables higher flow rates, so this form of cannulation is used when VAD support is used as a bridge to transplant, because ventricular injury is not a concern.
Ventricular Assist Device Flow and Function
Most VAD pumps used in children are either displacement pumps (pulsatile or pneumatic devices) or rotary pumps (continuous flow devices). The pulsatile or pneumatic pumps mimic the natural contraction (pumping action) of the heart. Flow rates depend on preload and the size of the external pump. Average flows for an infant-sized pump (12 or 15 mL) are 0.5 to 1.3 L/min and for a child-sized pump (25 or 30 mL) are 1.3 to 3.3 L/min.4 The external pump size can be changed to accommodate the child’s growth and increased stroke volume.
The most common continuous flow devices are axial or centrifugal pumps. Both types have a central rotor containing permanent magnets. Controlled electric currents that run through coils in the pump housing apply forces to the magnets causing the rotors to turn. Axial flow rates vary depending on the size of the device implanted. The child-sized device has been reported to provide an average flow of 0.3 to 2.5 L/min.4 The pediatric centrifugal Bio-Medicus Bio-pump (Medtronic, Inc. Minneapolis, MN) has both a 50- and 80-mL pump head size to accommodate infants <10 kg and children >10 kg, respectively. The 50-mL pump can provide flow up to 1.5 L/min and the 80-mL pump can provide >2 L/min.27
All VADs are preload dependent—the amount of blood returning to the heart is the amount of blood pumped to the body. The VAD is sensitive to impedance to flow, so hypertension and mechanical obstruction must be corrected. Both the LVAD and RVAD allow blood to bypass the failing ventricle. This decompresses that ventricle, decreases myocardial work, and reduces oxygen demand while maintaining adequate systemic perfusion to sustain end-organ function. VAD support has been shown to improve myocardial contractility. It also reverses beta receptor downregulation (documented to occur with heart failure), restoring myocardial response to the inotropic effects of adrenergic stimulation.48 VAD support can also normalize chamber geometry and reduce myocardial fibrosis, hypertrophy, and disruption in cytoskeletal proteins.10,34
Types of Ventricular Assist Device Pumps
Continuous Flow Pumps
Centrifugal Pump
The centrifugal pump is external, requires cannulation via a thoracotomy or sternotomy, and can be used for single or biventricular support. The most common centrifugal pump is the Bio-Medicus Bio-Pump (Medtronic, Inc. Minneapolis, Minn). The Bio-Pump uses two magnetically coupled, polycarbonate rotator cones that spin to create centrifugal force along a vertical axis.11 The rotors are shaped to accelerate the blood circumferentially and thus cause it to move toward the outer rim of the pump. The constrained vortex pump design creates subatmospheric pressure at the tip of the cone, establishing suction in the venous cannula.40 Blood enters at the apex of the cone and is ejected tangentially at the base of the cone (Fig. 7-3). The cone design retains any small air bubbles.
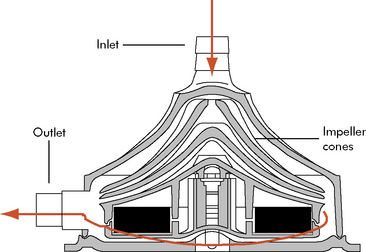
Fig. 7-3 Bio-Medicus centrifugal pump.
(From Karl TR, Horton SB, and Brizard C: Postoperative support with the centrifugal pump ventricular assist device (VAD). Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual 9:83–91, 2006.)
The pump output is proportional to revolutions per minute and is adjusted according to the venous return. Spins averaging 10,000 to 20,000 rpm will create a blood flow of 5 to 6 L/min in larger pumpheads.36 This type of pump can support neonates and older children with postoperative cardiac failure but competent lung function.36,58
The centrifugal VADs include Bio-Medicus Bio-pump, Levitronix CentriMag (Levotronix, Waltham, Mass.), RotaFlow (Jostra, Hirrlingen, Germany), and the Capiox Terumo (Terumo Cardiovascular Systems, Ann Arbor, Mich.). The Levitronix CentriMag is marketed in Europe, but available in the United Stated only as an investigational device.11 The advantage to this device is that it can be attached to cardiopulmonary bypass cannula already in place. However, cannula adaptors may be needed for smaller patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Be sure to check out the supplementary content available at
Be sure to check out the supplementary content available at