(MEL-fah-lan hy-droh-KLOR-eyed)
Alkeran, Evomela
Antineoplastic (alkylating agent/nitrogen mustard)
pH 6.5 to 7
Usual dose
Premedication:
Administration of a prophylactic antiemetic is recommended.
Multiple-myeloma palliative treatment (all products):
16 mg/M2 as an IV infusion over 15 to 20 minutes every 2 weeks for 4 doses. After recovery from toxicity, repeat dose every 4 weeks. Prednisone is administered concurrently.
Multiple-myeloma conditioning treatment (evomela):
100 mg/M2/day administered as an IV infusion over 30 minutes for 2 consecutive days (Day −3 and Day −2) prior to autologous stem cell transplantation (ASCT, Day 0). For patients who weigh more than 130% of their ideal body weight, body surface area should be calculated based on adjusted ideal body weight. Other unlabeled regimens are in use.
Pediatric dose
See Maternal/Child. All pediatric doses are unlabeled; consult literature.
Dose adjustments
All products:
For palliative treatment, manufacturer recommends reducing dose by 50% if BUN greater than 30 mg/dL.
Evomela:
No dose adjustment is necessary for conditioning treatment. For patients who weigh more than 130% of their ideal body weight, body surface area should be calculated based on adjusted ideal body weight.
Alkeran and generic:
Dose reduction based on blood counts at the nadir and on the day of treatment should be considered. In one clinical study, the following reductions based on cell count were followed: reduce dose by 25% if WBC between 3,000 and 4,000/mm3 and platelets between 75,000 and 100,000/mm3; by 50% if WBC between 2,000 and 3,000/mm3 and platelets between 50,000 and 75,000/mm3. Withhold dose for WBC below 2,000/mm3 and platelets below 50,000/mm3. ■ Lower-end initial doses may be indicated in the elderly. Consider impaired organ function and concomitant disease or drug therapy.
Dilution
Specific techniques required; see precautions.
All products are available in a 50-mg single-dose vial.
Alkeran and generic:
Reconstitution to completion of administration must take place within 60 minutes due to instability of melphalan (rapid hydrolysis). Rapidly inject 10 mL of supplied diluent into vial using a sterile needle (20-gauge or larger needle diameter) and syringe. Shake vigorously until a clear solution results (5 mg/mL). Use only clear solutions. Immediately, further dilute in NS to a concentration not greater than 0.45 mg/mL. Drug is very unstable and may begin to deteriorate within 30 minutes.
Evomela:
Reconstitute with 8.6 mL of NS to make a 5 mg/mL solution. The NS should be assisted or pulled into the vial by a partial vacuum. Do not use if this vacuum is not present. Calculate the required volume needed for the patient dose. Withdraw this volume from the vial and inject into the appropriate volume of NS to create a solution with a final concentration of 0.45 mg/mL.
Filters:
No data available from manufacturer. Another source indicates minimal adsorption with several types of filters from 0.2 to 0.45 microns in size.
Storage:
All products:
Store at CRT in carton to protect from light.
Alkeran and generic:
Keep time between reconstitution/dilution and administration to a minimum because reconstituted and diluted solutions are unstable. Complete administration within 60 minutes of reconstitution. Reconstituted solution will precipitate if refrigerated. Do not refrigerate.
Evomela:
Reconstituted solution is stable for 1 hour at RT and 24 hours if refrigerated. Diluted solution is stable for 4 hours at RT in addition to the 1 hour following reconstitution.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Evomela:
Manufacturer states that Evomela should not be mixed with other melphalan hydrochloride for injection drug products.
Alkeran and generic:
One source suggests the following compatibilities:
Y-site:
Acyclovir (Zovirax), amikacin, aminophylline, ampicillin, aztreonam (Azactam), bleomycin (Blenoxane), bumetanide, buprenorphine (Buprenex), butorphanol (Stadol), calcium gluconate, carboplatin (Paraplatin), carmustine (BiCNU), caspofungin (Cancidas), cefazolin (Ancef), cefotaxime (Claforan), cefotetan, ceftazidime (Fortaz), ceftriaxone (Rocephin), cefuroxime (Zinacef), cisplatin, clindamycin (Cleocin), cyclophosphamide (Cytoxan), cytarabine (ARA-C), dacarbazine (DTIC), dactinomycin (Cosmegen), daunorubicin (Cerubidine), dexamethasone (Decadron), diphenhydramine (Benadryl), doxorubicin (Adriamycin), doxycycline, droperidol (Inapsine), enalaprilat (Vasotec IV), etoposide (VePesid), famotidine (Pepcid IV), filgrastim (Neupogen), fluconazole (Diflucan), fludarabine (Fludara), fluorouracil (5-FU), furosemide (Lasix), gallium nitrate (Ganite), ganciclovir (Cytovene IV), gentamicin, granisetron (Kytril), heparin, hydrocortisone sodium succinate (Solu-Cortef), hydromorphone (Dilaudid), idarubicin (Idamycin), ifosfamide (Ifex), imipenem-cilastatin (Primaxin), lorazepam (Ativan), mannitol, mechlorethamine (nitrogen mustard), meperidine (Demerol), mesna (Mesnex), methotrexate, methylprednisolone (Solu-Medrol), metoclopramide (Reglan), metronidazole (Flagyl IV), mitomycin (Mutamycin), mitoxantrone (Novantrone), morphine, nalbuphine, ondansetron (Zofran), pentostatin (Nipent), potassium chloride (KCl), prochlorperazine (Compazine), promethazine (Phenergan), ranitidine (Zantac), sodium bicarbonate, streptozocin (Zanosar), sulfamethoxazole/trimethoprim, teniposide (Vumon), thiotepa, ticarcillin/clavulanate (Timentin), tobramycin, vancomycin, vinblastine, vincristine, vinorelbine (Navelbine), zidovudine (AZT, Retrovir).
Rate of administration
Do not administer directly into a peripheral vein.
Alkeran and generic:
Keep the time from reconstitution to dilution to administration to a minimum. Drug is very unstable and may begin to deteriorate within 30 minutes. Complete administration within 60 minutes of reconstitution. Manufacturer recommends administration by injecting slowly into a fast-running IV solution via an injection port or via a central venous line. In cases of poor peripheral venous access, consideration should be given to use of a central venous line.
Evomela:
Inject slowly into a fast-running IV infusion via a central venous access line.
Palliative treatment (all products):
A single dose as an IV infusion over 15 to 20 minutes.
Conditioning treatment (evomela):
Actions
A phenylalanine derivative of nitrogen mustard that is a bifunctional alkylating antineoplastic agent. Cytotoxicity is related to the extent of its interstrand cross-linking with DNA. Not dependent on cell cycle phase. Active against both resting and dividing tumor cells. Binding to plasma proteins (primarily albumin) ranges from approximately 50% to 90%. Approximately 30% is irreversibly bound to plasma proteins. Eliminated from plasma primarily by chemical hydrolysis. Half-life is approximately 75 minutes. About 10% excreted as unchanged drug in urine.
Indications and uses
All products:
Palliative treatment of patients with multiple myeloma when oral therapy is not appropriate.
Evomela:
High-dose conditioning treatment prior to hematopoietic progenitor (stem) cell transplantation in patients with multiple myeloma.
Unlabeled uses:
Alkeran and generic:
Conditioning regimen before autologous hematopoietic stem cell transplantation (HSCT). ■ Component of combination therapy to treat relapsed, resistant Hodgkin’s lymphomas. ■ Pediatric rhabdomyosarcoma. ■ HSCT for pediatric neuroblastoma, pediatric hematologic malignancies, and Ewing sarcoma.
Contraindications
All products:
History of serious hypersensitivity to melphalan.
Alkeran and generic:
Patients whose disease has demonstrated prior resistance to melphalan.
Precautions
Follow guidelines for handling cytotoxic agents. See Appendix A, p. 1331. ■ Administered by or under the direction of the physician specialist, with facilities for monitoring the patient and responding to any medical emergency. ■ Severe myelotoxicity with resulting infection or bleeding may occur. Trials comparing IV to oral melphalan have shown more myelosuppression with the IV formulation. ■ For patients receiving Evomela as part of a conditioning regimen, myeloablation occurs in all patients. Do not begin the conditioning regimen unless a stem cell product is available for rescue. Monitor blood counts and provide supportive care for infections, anemia, and thrombocytopenia until there is adequate hematopoietic recovery. ■ When melphalan is being administered for palliative treatment, use extreme caution in patients whose bone marrow is compromised by or recovering from previous radiation or chemotherapy. ■ GI toxicity, including nausea, vomiting, mucositis, and diarrhea, has been reported with both indications. May occur in over 50% of patients receiving the conditioning regimen. Use prophylactic antiemetic therapy and provide supportive care for GI toxicity. ■ Hepatotoxicity ranging from abnormal liver function tests to hepatitis and hepatic venoocclusive disease has been reported. ■ Hypersensitivity reactions, including anaphylaxis, have been reported. ■ Do not abandon treatment prematurely when used for palliative treatment. Improvement may continue slowly over many months with repeated courses. ■ Patients with an elevated BUN had a greater incidence of severe bone marrow suppression. ■ Produces chromosomal aberrations in vitro and in vivo. Should be considered potentially leukemogenic in humans. Secondary malignancies, including acute nonlymphocytic leukemia, myeloproliferation syndrome, and carcinoma have been reported in patients treated with alkylating agents, including melphalan. ■ See Drug/Lab Interactions.
Monitor:
Determine absolute patency of vein. A stinging or burning sensation indicates extravasation; cellulitis and tissue necrosis may result. Discontinue injection; use another vein; see Rate of Administration. ■ Frequently monitor platelet count, hemoglobin, white blood cell count, and differential. For palliative treatment, monitoring is indicated before each dose as well as between doses to determine optimal dose and avoid toxicity. Nadirs occur 2 to 3 weeks after treatment; recovery should occur in 4 to 5 weeks. ■ Severe myelosuppression can occur with effective doses. Withhold further doses until blood cell counts have recovered if thrombocytopenia and/or leukopenia occur. ■ Monitor renal function before and during therapy. ■ Monitor liver function tests. ■ Hypersensitivity reactions may occur with initial treatment or may be delayed and occur after multiple courses. Observe closely. ■ Observe closely for all signs of infection, bleeding, or symptomatic anemia and provide supportive care as indicated. Prophylactic antibiotics may be indicated pending results of C/S in a febrile neutropenic patient. ■ Prophylactic antiemetics may reduce nausea and vomiting and increase patient comfort. ■ Nutritional support and analgesics may be required in patients experiencing Grade 3 or 4 mucositis. ■ Monitor for thrombocytopenia (platelet count less than 50,000/mm3). Initiate precautions to prevent excessive bleeding (e.g., inspect IV sites, skin, and mucous membranes; use extreme care during invasive procedures; test urine, emesis, stool, and secretions for occult blood). ■ See Drug/Lab Interactions.
Patient education:
Avoid pregnancy. Females of reproductive potential and males with female sexual partners of reproductive potential should use effective contraception methods during and after treatment. ■ Treatment with melphalan may result in temporary or permanent infertility. ■ Promptly report IV site burning or stinging. ■ Acute side effects are related to bone marrow suppression, hypersensitivity reactions, GI toxicity, and pulmonary toxicity. Promptly report S/S of a hypersensitivity reaction or any potential side effects (e.g., bleeding, cough, fever, mucositis, rash). ■ Routine laboratory monitoring is required. ■ Major long-term toxicities are related to infertility and secondary malignancy. Discuss risks with health care provider. ■ See Appendix D, p. 1333.
Maternal/child:
Avoid pregnancy. Based on its mechanism of action, melphalan can cause fetal harm, including teratogenicity and/or embryo-fetal lethality. ■ Discontinue breast-feeding. ■ Safety and effectiveness for use in pediatric patients not established; see Unlabeled Uses.
Elderly:
Response similar to that seen in younger adults. However, a greater incidence of engraftment syndrome was observed in older patients. ■ See Dose Adjustments.
Drug/lab interactions
Coadministration with cyclosporine (Sandimmune) may result in acute renal failure. May occur after the first dose of each drug. ■ Renal dysfunction induced by cisplatin may lead to decreased clearance and increased toxicity of melphalan. ■ Threshold for lung toxicity associated with carmustine (BiCNU) may be reduced with concurrent use. ■ Interferon alfa may decrease serum concentrations of melphalan. ■ Use with nalidixic acid (NegGram) may cause severe hemorrhagic necrotic enterocolitis in pediatric patients. ■ Do not administer live virus vaccines to immunocompromised patients receiving antineoplastic agents.
Side effects
The most common adverse reactions (occurring in at least 50% of patients) include anemia; decreased lymphocyte count, neutrophil count, platelet count, and white blood cell count; diarrhea; fatigue; hypokalemia; nausea; and vomiting. The most common serious adverse reactions were febrile neutropenia, fever, hematochezia, and renal failure. Reversible bone marrow suppression (e.g., anemia, leukopenia, thrombocytopenia) is dose-limiting. Irreversible bone marrow failure has been reported. Abdominal pain, alopecia, constipation, decreased appetite, dizziness, elevated BUN, hemolytic anemia, hepatic toxicity (including venoocclusive disease), hypersensitivity reactions (e.g., anaphylaxis, bronchospasm, dyspnea, dysgeusia, dyspepsia, edema, hypotension, mucosal inflammation, peripheral edema, pruritus, rash, urticaria, tachycardia), hypophosphatemia, interstitial pneumonitis, pulmonary fibrosis, secondary malignancies (long-term use), skin ulceration at injection site, stomatitis, and vasculitis have occurred.
Overdose:
Adult respiratory distress syndrome, bone marrow suppression (severe), cholinomimetic effects (e.g., bradycardia, increased peristalsis and salivation, incontinence), convulsions, decreased consciousness, hyponatremia (inappropriate secretion of ADH), GI toxicity (colitis, diarrhea, GI bleed, mucositis, nausea, stomatitis, and vomiting), muscular paralysis, and nephrotoxicity.
Antidote
Keep physician informed of all side effects. Close monitoring of bone marrow may prevent most serious and potentially fatal side effects. WBC and platelet count nadirs occur 2 to 3 weeks after treatment with recovery in 4 to 5 weeks. Withhold further doses until blood cell counts have recovered if leukopenia or thrombocytopenia occur. There is no specific antidote, but adequate supportive care including administration of whole blood products (e.g., packed RBCs, platelets, leukocytes) and/or blood modifiers (e.g., darbepoetin alfa [Aranesp], epoetin alfa [Epogen], filgrastim [Neupogen, Zarxio], pegfilgrastim [Neulasta], sargramostim [Leukine]) may be indicated to treat bone marrow toxicity. Appropriate antibiotics may be indicated. Monitor closely until recovery (6 weeks or more). Discontinue the infusion for severe hypersensitivity reactions and treat with antihistamines, corticosteroids, pressor agents, or volume expanders; do not readminister melphalan (IV or oral). Hemodialysis probably not effective in overdose.
Meperidine hydrochloride
(meh-PER-ih-deen hy-droh-KLOR-eyed)
Demerol, Demerol HCl, Meperidine HCl PF
Opioid analgesic (agonist)
Anesthesia adjunct
pH 3.5 to 6
Usual dose
Determination of dose should be based on severity of pain and patient response.
IV injection:
5 to 10 mg every 5 minutes as needed. Do not exceed 600 mg/24 hr. IV dosing in acute pain should be limited to 48 hours or less; see Precautions.
Supplement anesthesia:
1 to 10 mg/mL dilution is usually used. Titrate under the direct observation and control of the anesthesiologist. Dose dependent on premedication, type of anesthesia, type and duration of procedure, and patient’s condition.
Treatment or prevention of shaking chills (unlabeled):
0.5 mg/kg 20 minutes before shaking chills are expected to begin or 50 mg after onset of chills. Up to 150 mg has been required within 30 minutes if administered after onset.
Pediatric dose
1 mg/kg/dose; see Maternal/Child.
Dose adjustments
Reduced dose may be required in the elderly or debilitated, in hepatic or renal disease, or in numerous other disease entities; see Precautions. ■ Doses appropriate for the general population may cause serious respiratory depression in vulnerable patients. ■ Increase doses as required if analgesia is inadequate, tolerance develops, or pain severity increases. The first sign of tolerance is usually a reduced duration of effect. ■ Decrease dose by 25% to 50% when administered concomitantly with phenothiazines and other centrally acting medications (e.g., sedatives). ■ See Drug/Lab Interactions.
Dilution
IV injection:
May be given undiluted; however, further dilution with 5 mL of SWFI, NS, or other IV solutions to facilitate titration is appropriate; see chart on inside back cover.
Filters:
No data available from manufacturer.
Storage:
Before use, store at CRT protected from light. Do not freeze.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
One source suggests the following compatibilities:
Additive:
Cefazolin (Ancef), dobutamine, metoclopramide (Reglan), ondansetron (Zofran), sodium bicarbonate, succinylcholine, verapamil.
Y-site:
Acetaminophen (Ofirmev), acyclovir (Zovirax), amifostine (Ethyol), amikacin, ampicillin, ampicillin/sulbactam (Unasyn), anidulafungin (Eraxis), aztreonam (Azactam), bivalirudin (Angiomax), bumetanide, caspofungin (Cancidas), cefazolin (Ancef), cefotaxime (Claforan), cefotetan, cefoxitin (Mefoxin), ceftaroline (Teflaro), ceftazidime (Fortaz), ceftriaxone (Rocephin), cefuroxime (Zinacef), chloramphenicol (Chloromycetin), cisatracurium (Nimbex), cladribine (Leustatin), clindamycin (Cleocin), dexamethasone (Decadron), dexmedetomidine (Precedex), digoxin (Lanoxin), diltiazem (Cardizem), diphenhydramine (Benadryl), dobutamine, docetaxel (Taxotere), dopamine, doripenem (Doribax), doxycycline, droperidol (Inapsine), erythromycin (Erythrocin), etoposide phosphate (Etopophos), famotidine (Pepcid IV), fenoldopam (Corlopam), filgrastim (Neupogen), fluconazole (Diflucan), fludarabine (Fludara), furosemide (Lasix), gallium nitrate (Ganite), gemcitabine (Gemzar), gentamicin, granisetron (Kytril), heparin, hetastarch in electrolytes (Hextend), hydrocortisone sodium succinate (Solu-Cortef), 6% hydroxyethyl starch (Voluven), insulin (regular), labetalol, lidocaine, linezolid (Zyvox), magnesium sulfate, melphalan (Alkeran), methyldopate, methylprednisolone (Solu-Medrol), metoclopramide (Reglan), metoprolol (Lopressor), metronidazole (Flagyl IV), ondansetron (Zofran), oxacillin (Bactocill), oxaliplatin (Eloxatin), oxytocin (Pitocin), paclitaxel (Taxol), palonosetron (Aloxi), pemetrexed (Alimta), penicillin G potassium, piperacillin/tazobactam (Zosyn), potassium chloride (KCl), propofol (Diprivan), propranolol, ranitidine (Zantac), remifentanil (Ultiva), sargramostim (Leukine), sulfamethoxazole/trimethoprim, teniposide (Vumon), thiotepa, ticarcillin/clavulanate (Timentin), tobramycin, vancomycin, verapamil, vinorelbine (Navelbine).
Rate of administration
IV injection:
A single dose over 4 to 5 minutes. Frequently titrated according to symptom relief and respiratory rate. Rapid IV administration increases the possibility of hypotension and respiratory depression.
Actions
A synthetic narcotic analgesic and descending CNS depressant, similar to morphine. Has multiple actions; the most prominent involve the CNS and organs composed of smooth muscle. Principal actions of therapeutic value are analgesia and sedation. May produce less smooth muscle spasm, constipation, and depression of the cough reflex than equianalgesic doses of morphine. A parenteral dose of meperidine 60 to 80 mg is approximately equivalent in analgesic effect to 10 mg of morphine. Onset of action occurs in about 5 minutes and lasts for about 2 to 4 hours. Readily absorbed and distributed throughout the body. Metabolized to an active, toxic metabolite (normeperidine) in the liver, its extended half-life (15 to 30 hours) may lead to cumulative effects. Excreted in the urine. Crosses the placental barrier. Secreted in breast milk.
Indications and uses
Short-term relief of moderate to severe pain. ■ Preoperative medication. ■ Support of anesthesia. ■ Obstetric analgesia.
Unlabeled uses:
Treatment or prevention of shaking chills (rigors) caused by some medications (e.g., amphotericin B [all formulations], aldesleukin [Proleukin]). ■ Treatment of postoperative shivering.
Contraindications
Hypersensitivity to meperidine, patients who have received MAO inhibitors (e.g., selegiline [Eldepryl]) in the previous 14 days.
Precautions
Use of meperidine as a first-line analgesic for pain is discouraged due to its short duration of action and the risk of accumulation of its toxic metabolite, normeperidine. Accumulation of normeperidine may increase the risk of toxicity (e.g., seizures). If its use in acute pain (in patients without renal or CNS disease) cannot be avoided, the American Pain Society and ISMP recommend limiting treatment to 48 hours or less and not exceeding 600 mg/24 hr. ■ Use with caution in glaucoma, head injuries, increased intracranial pressure (elevates spinal fluid pressure), asthma, chronic obstructive pulmonary disease, decreased respiratory reserve or respiratory depression, supraventricular tachycardia, convulsions, acute abdominal conditions before diagnosis, the elderly and debilitated, and hepatic or renal insufficiency. ■ Use with caution and in reduced doses in patients receiving concurrent therapy with other narcotic analgesics, general anesthetics, phenothiazines (e.g., promethazine [Phenergan], prochlorperazine [Compazine]), sedative-hypnotics (including barbiturates [e.g., phenobarbital]), tricyclic antidepressants (e.g., imipramine [Tofranil]), and other CNS depressants, including alcohol. Use may result in respiratory depression, hypotension, and profound sedation or coma. See Drug/Lab Interactions. ■ Use with caution in patients with renal dysfunction; normeperidine may accumulate, resulting in increased CNS toxicity. ■ May cause severe hypotension in postoperative patients or in any patient whose ability to maintain blood pressure has been compromised by depleted blood volume or concomitant administration of drugs that can cause hypotension (e.g., anesthetics, phenothiazines). ■ Use with caution in patients with sickle cell anemia, hypothyroidism, Addison’s disease, pheochromocytoma, and prostatic hypertrophy or urethral stricture. Reduced doses may be indicated; see Dose Adjustments. ■ Cough reflex may be suppressed. ■ Morphine is usually preferred for pain during an acute MI. ■ IM route frequently used. Frequent IM injections may lead to severe fibrosis of muscle tissue. ■ Do not use in patients for any long-term pain relief (e.g., cancer). ■ Psychological and physical dependence and tolerance may develop with repeated administration.
Monitor:
Oxygen, controlled respiratory equipment, and naloxone must always be available. ■ Observe patient frequently to continuously based on amount of dose and monitor vital signs. ■ Assess baseline pain, then assess pain with vital signs. Reassess after administration of meperidine and adjust dose or interval as required. ■ Keep patient supine; orthostatic hypotension and fainting may occur; less likely with continuous low doses, but observe closely during ambulation. ■ Uncontrolled pain causes sleep deprivation, decreases pain threshold, and increases pain. When pain is finally controlled, expect the patient to sleep more until recovery from sleep deprivation. ■ With use the active metabolite normeperidine accumulates to toxic levels; will lower seizure threshold. Monitor for twitching, jerking, shaky hands, tremors; may lead to grand mal seizure. ■ Laxatives with or without stool softeners may be required to avoid constipation and fecal impaction. Maintain adequate hydration.
Patient education:
Avoid alcohol or other CNS depressants (e.g., barbiturates, benzodiazepines [e.g., diazepam (Valium)]). ■ May cause blurred vision, dizziness, or drowsiness; use caution in tasks that require alertness. ■ Request assistance with ambulation. ■ May be habit forming.
Maternal/child:
Category C: safety for use before labor not established. ■ Use during delivery may cause depression of respiration and psychophysiologic functions in the newborn requiring resuscitation. ■ May cause serious adverse reactions in nursing infants; discontinue breast-feeding or discontinue meperidine. ■ Not recommended for IV use in pediatric patients but is used. Consider risk versus benefit before use in neonates or young infants. Rate of elimination is slower in neonates and young infants compared to older children or adults. May be more sensitive to effects (e.g., respiratory depression) and may cause paradoxical excitation.
Elderly:
See Dose Adjustments and Precautions. ■ Elimination rate is slower than in younger adults. ■ May be more sensitive to effects (e.g., respiratory depression, constipation, urinary retention). ■ Lower doses may provide effective analgesia. ■ Consider age-related organ impairment.
Drug/lab interactions
Potentiated by acyclovir (Zovirax), anticholinergics, cimetidine (Tagamet), tricyclic antidepressants (e.g., imipramine [Tofranil]), isoniazid (INH), neostigmine, neuromuscular blocking agents (e.g., atracurium [Tracrium]), phenothiazines, general anesthetics, other narcotic analgesics, and CNS depressants including alcohol. Side effects (e.g., CNS depression, constipation, hypotension) may be additive. Reduced dosage of both drugs may be indicated. ■ Do not use with MAO inhibitors (e.g., selegiline [Eldepryl]); may cause cardiovascular collapse. ■ Avoid concurrent use with sibutramine (Meridia); may precipitate serotonin syndrome (e.g., altered consciousness, CNS irritability, motor weakness, myoclonus, shivering). ■ Mixed agonists/antagonists (e.g., pentazocine [Talwin], nalbuphine, butorphanol [Stadol]) may decrease analgesic effect of meperidine and/or precipitate withdrawal symptoms. ■ Concurrent use with protease inhibitors (e.g., saquinavir [Invirase], ritonavir [Norvir]) not recommended. May increase meperidine serum concentrations and increase the risk of side effects, including seizures and cardiac arrhythmias. ■ Hydantoins (e.g., phenytoin [Dilantin]) may increase metabolism and decrease the half-life of meperidine; however, increased normeperidine levels have been seen. ■ Metabolism increased and analgesic effects may be decreased or delayed in smokers.
Side effects
Dizziness, flushing, light-headedness, nausea, postural hypotension, rash, restlessness, sedation, sweating, syncope, vomiting. Side effects associated with histamine release, convulsions, and constipation may be more common with meperidine than with most other narcotic analgesics.
Major:
Apnea, cardiac arrest, cardiovascular collapse, cold and clammy skin, convulsions, dilated pupils, hypersensitivity reactions (e.g., anaphylaxis, pruritus), normeperidine toxicity (jerking, tremor, twitching, shaky hands, grand mal seizure), respiratory depression, shock, tremor.
Antidote
With increasing severity of minor side effects or onset of any major side effect, discontinue the drug and notify the physician. A patent airway, artificial respiration, oxygen therapy, and other symptomatic treatment must be instituted promptly. Naloxone hydrochloride will reverse cardiovascular, CNS, and respiratory reactions. In patients who are physically dependent on narcotics, either avoid the use of a narcotic antagonist or use extreme caution and doses as small as one-fifth to one-tenth of the usual initial dose to avoid precipitating an acute withdrawal syndrome. In all patients, adjust and titrate the dose of a narcotic antagonist to reverse side effects without reversing pain control. Avoid total reversal of pain control. Resuscitate as necessary.
Meropenem
(mer-oh-PEN-em)
Merrem I.V.
Antibacterial (carbapenem)
pH 7.3 to 8.3
Usual dose
Dose ranges from 500 mg to 2 Gm and depends on type and severity of infection.
Complicated skin and skin structure infections in adults and pediatric patients weighing 50 kg or more:
500 mg every 8 hours. Increase to 1 Gm every 8 hours in complicated skin and skin structure infections caused by P. aeruginosa.
Intra-abdominal infections in adults and pediatric patients weighing 50 kg or more:
1 Gm every 8 hours.
Febrile neutropenia in adults and pediatric patients weighing 50 kg or more (unlabeled):
1 Gm every 8 hours.
Meningitis (unlabeled in adult patients):
2 Gm every 8 hours.
Burkholderia infections (melioidosis [unlabeled]):
1 Gm every 8 hours.
Mild to moderate infection, other severe infections (unlabeled):
500 mg to 1 Gm every 8 hours.
Complicated urinary tract infections (unlabeled):
500 mg to 1 Gm every 8 hours.
Pediatric dose
30 to 120 mg/kg/day divided (10 to 40 mg) every 8 hours depending on type and severity of infection. Maximum dose is 6 Gm/day. See Maternal/Child.
Complicated skin and skin structure infections in pediatric patients over 3 months of age:
10 mg/kg every 8 hours. Maximum single dose every 8 hours is 500 mg. Increase dose to 20 mg/kg every 8 hours in complicated skin and skin structure infections caused by P. aeruginosa. Maximum single dose every 8 hours is 1 Gm.
Intra-abdominal infections in pediatric patients over 3 months of age:
20 mg/kg every 8 hours. Maximum single dose every 8 hours is 1 Gm.
Intra-abdominal infections in pediatric patients under 3 months of age:
Dose is based on gestational age (GA) and postnatal age (PNA).
Infants under 32 weeks GA and PNA under 2 weeks:
20 mg/kg every 12 hours.
Infants under 32 weeks GA and PNA 2 weeks and older:
20 mg/kg every 8 hours.
Infants 32 weeks and older GA and PNA under 2 weeks:
20 mg/kg every 8 hours.
Infants 32 weeks and older GA and PNA 2 weeks and older:
30 mg/kg every 8 hours.
Meningitis in pediatric patients over 3 months of age:
40 mg/kg every 8 hours. Maximum single dose every 8 hours is 2 Gm.
Febrile neutropenia in pediatric patients 3 months of age and older and less than 50 kg (unlabeled):
20 mg/kg every 8 hours.
Dose adjustments
Reduced dose required if CrCl is less than 51 mL/min based on the following chart.
| Meropenem Recommended IV Dosage Schedule for Adults with Impaired Renal Function | ||
| Creatinine Clearance (mL/min) | Dose (dependent on type of infection) | Dosing Interval |
| 26-50 mL/min | Recommended dose | Every 12 hours |
| 10-25 mL/min | One half recommended dose | Every 12 hours |
| <10 mL/min | One half recommended dose | Every 24 hours |
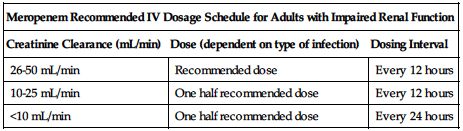
Consult package insert or front matter of this text for formula to convert SCr to CrCl. ■ No dose adjustment necessary in impaired hepatic function. ■ Reduced dose may be required in the elderly based on decreased renal function. ■ Information is inadequate for use in patients on hemodialysis or peritoneal dialysis. ■ No experience in pediatric patients with renal impairment.
Dilution
Injection:
Reconstitute each 500 mg with 10 mL SWFI (1 Gm with 20 mL). Yields 50 mg/mL. Shake to dissolve and let stand until clear. May be given as an IV injection or further diluted with compatible infusion solutions (see Infusion and Compatibility).
Infusion:
Available as infusion vials that may be directly reconstituted with a compatible solution and then infused. Concentration may range from 1 to 20 mg/mL.
Storage:
Store unopened vials (dry powder) at RT (20° to 25° C [68° to 77° F]). Use of freshly prepared solutions preferred. Injection vials reconstituted with SWFI for bolus administration (up to 50 mg/mL) may be stored for up to 3 hours at up to 25° C (77° F) or for 13 hours at up to 5° C (41° F). Solutions prepared for infusion with NS (concentrations ranging from 1 to 20 mg/mL) may be stored for 1 hour at up to 25° C (77° F) or for 15 hours at up to 5° C (41° F). Solutions prepared for infusion with D5W (concentrations ranging from 1 to 20 mg/mL) should be used immediately. Do not freeze.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer states, “Meropenem should not be mixed or physically added to solutions containing other drugs; compatibility not established.”
One source suggests the following compatibilities:
Solution:
Compatible under specific conditions (see Storage) with NS, D5W, D10W, D5NS, D5/1/4NS, KCl 0.15% in D5W, Na Bicarbonate 0.02% in D5W, D5 in Normosol-M, D5LR, D21/2 in 1/2NS, Mannitol 2.5%, R, LR, Na Lactate 1/6 M, Na Bicarbonate 5%.
Additive:
Not recommended by manufacturer.
Acyclovir (Zovirax), aminophylline, atropine, dexamethasone (Decadron), dobutamine, dopamine, doxycycline, enalaprilat (Vasotec IV), fluconazole (Diflucan), furosemide (Lasix), gentamicin, heparin, insulin (regular), magnesium sulfate, metoclopramide (Reglan), morphine, norepinephrine (Levophed), ondansetron (Zofran), phenobarbital (Luminal), ranitidine (Zantac), vancomycin, zidovudine (AZT, Retrovir).
Y-site:
Acyclovir (Zovirax), aminophylline, anidulafungin (Eraxis), atropine, calcium gluconate, caspofungin (Cancidas), cyclosporine (Sandimmune), dexamethasone (Decadron), digoxin (Lanoxin), diphenhydramine (Benadryl), docetaxel (Taxotere), doxycycline, enalaprilat (Vasotec IV), fluconazole (Diflucan), furosemide (Lasix), gentamicin, heparin, insulin (regular), linezolid (Zyvox), metoclopramide (Reglan), milrinone (Primacor), morphine, norepinephrine (Levophed), ondansetron (Zofran), phenobarbital (Luminal), potassium chloride (KCl), telavancin (Vibativ), vancomycin, vasopressin, zidovudine (AZT, Retrovir).
Rate of administration
IV injection in adults and pediatric patients over 3 months of age:
A single dose (up to 1 Gm [20 mL] after dilution) over 3 to 5 minutes.
Intermittent infusion in adults and pediatric patients over 3 months of age:
A single dose over 15 to 30 minutes.
Intermittent infusion in pediatric patients under 3 months of age:
A single dose over 30 minutes.
Extended infusion (unlabeled):
0.5 to 2 Gm over 3 hours every 8 hours.
Actions
A synthetic, broad-spectrum, carbapenem antibiotic. Bactericidal to selected gramnegative, gram-positive, and anaerobic organisms. Bactericidal activity results from the inhibition of cell wall synthesis. Readily penetrates the cell wall of susceptible organisms to reach penicillin-binding protein targets. Has significant stability to hydrolysis by penicillinases and cephalosporinases produced by gram-positive and gram-negative bacteria. Peak plasma concentrations reached by the end of an infusion. Penetrates well into most body fluids and tissues, including cerebrospinal fluid. Peak fluid and tissue concentrations reached in 0.5 to 1.5 hours. Minimal protein binding. Elimination half-life averages 1 hour in adults, 1.5 hours in pediatric patients age 3 months to 2 years, and 2.7 hours in infants under 3 months of age. 70% recovered as unchanged drug in urine within 12 hours. Not yet known if it crosses the placental barrier. Secreted in breast milk.
Indications and uses
Indicated as single-agent therapy for treatment of the specific infections caused by susceptible organisms. Is useful as presumptive therapy in the indicated infections before identification of the causative organism because of its broad spectrum of activity. Treatment of intra-abdominal infections (e.g., complicated appendicitis, peritonitis) in adults and pediatric patients. ■ Treatment of complicated skin and skin structure infections in adults and pediatric patients 3 months of age and older. ■ Treatment of bacterial meningitis in pediatric patients 3 months of age and older. Efficacy of meropenem as monotherapy in the treatment of meningitis caused by penicillin-nonsusceptible isolates of Streptococcus pneumoniae has not been established. Meropenem has been found to be effective in eliminating concurrent bacteremia in association with bacterial meningitis.
Unlabeled uses:
Empiric anti-infective therapy in febrile neutropenic patients. ■ Meningitis in adults. ■ Septicemia. ■ Complicated urinary tract infections caused by susceptible bacteria. ■ Respiratory tract infections. ■ Alternate or concomitant therapy in Acinetobacter, anthrax, Bacillus cereus, melioidosis caused by Burkholderia pseudomallei, Campylobacter fetus, Capnocytophaga canimorsus, Clostridium perfringens, glanders caused by Burkholderia mallei, Nocardia, and Rhodococcus equi infections.
Contraindications
History of hypersensitivity to meropenem, its components, any other carbapenem antibiotic (e.g., imipenem-cilastatin [Primaxin]), or patients with demonstrated anaphylaxis to beta-lactams; see Precautions.
Precautions
Specific sensitivity studies are indicated to determine susceptibility of the causative organism to meropenem. ■ To reduce the development of drug-resistant bacteria and maintain its effectiveness, meropenem should be used to treat or prevent only those infections proven or strongly suspected to be caused by bacteria. ■ Serious and occasionally fatal hypersensitivity reactions have been reported in patients receiving therapy with beta-lactams (e.g., penicillins, cephalosporins, carbapenems). More likely in patients with a history of sensitivity to multiple allergens; obtain a careful history. Crosssensitivity is possible. ■ Seizures and other adverse CNS reactions have been reported. Occurred most commonly in patients with a history of CNS disorders (e.g., brain lesions, history of seizures) or with bacterial meningitis and/or compromised renal function. Use extreme caution; continue administration of anticonvulsants in patients with known seizure disorders. ■ Use with caution in patients with impaired renal function; thrombocytopenia may occur and the incidence of heart failure, kidney failure, seizures, and shock may be increased; see Dose Adjustments. ■ May have cross-resistance with strains resistant to other carbapenems (e.g., imipenem-cilastatin [Primaxin]). ■ Localized clusters of infections resulting from carbapenem-resistant bacteria have been reported in some regions. ■ Has the potential for neuromotor impairment (e.g., headaches, paresthesias, seizures) that can interfere with mental alertness and/or cause motor impairment; see Patient Education. ■ Avoid prolonged use of drug; superinfection caused by overgrowth of nonsusceptible organisms may result. ■ Clostridium difficile–associated diarrhea (CDAD) has been reported. May range from mild diarrhea to fatal colitis. Consider in patients who present with diarrhea during or after treatment with meropenem. ■ See Drug/Lab Interactions.
Monitor:
Anaphylaxis has been reported. Emergency equipment must always be available. ■ Monitor infusion site for inflammation and/or extravasation. May cause thrombophlebitis. ■ Monitor for S/S of CNS reactions (e.g., focal tremors, myoclonus, seizures). ■ Monitor renal, hepatic, and hemopoietic systems in prolonged therapy. ■ Each 1 Gm contains 3.92 mEq of sodium; monitoring of electrolytes may be indicated. ■ See Drug/Lab Interactions and Side Effects.
Patient education:
Report any itching, rash, shortness of breath, or twitching sensation immediately. ■ Report any burning, pain, or stinging at injection site. ■ Promptly report diarrhea or bloody stools that occur during treatment or up to several months after an antibiotic has been discontinued; may indicate CDAD and require treatment. ■ Patients with a history of seizures should review medication profile with physician before taking meropenem; see Drug/Lab Interactions. ■ May interfere with mental alertness and/or cause motor impairment. Do not operate machinery or drive until tolerance is established.
Maternal/child:
Category B: safety for use in pregnancy not established; use only if clearly needed. ■ Use caution during breast-feeding. ■ Safety and effectiveness established for pediatric patients 3 months of age and older with complicated skin and skin structure infections or bacterial meningitis and for pediatric patients of all ages with complicated intra-abdominal infections. ■ Safety data are limited to support the administration of a 40 mg/kg (up to a maximum of 2 Gm) bolus dose.
Elderly:
Consider age-related renal impairment; plasma clearance is decreased with decreased renal function; see Dose Adjustments. Monitoring of renal function is suggested. Response is similar to that seen in younger patients; however, greater sensitivity in the elderly cannot be ruled out. See Precautions.
Drug/lab interactions
Carbapenems may reduce serum valproic acid concentrations to subtherapeutic levels, resulting in a loss of seizure control. Monitor valproic acid levels. Consider alternative antibacterial therapy. If administration of meropenem is necessary, supplemental anticonvulsant therapy should be considered. ■ Probenecid inhibits renal excretion and increases serum levels of meropenem, extending its half-life and increasing systemic exposure; coadministration is not recommended. ■ May be synergistic with aminoglycosides against some isolates of Pseudomonas aeruginosa.
Side effects
Toxicity rate is usually low.
Pediatric patients:
The types of clinical adverse effects seen in pediatric patients are similar to those seen in adults. The most commonly reported adverse effects included diarrhea, diaper area moniliasis, glossitis, oral moniliasis, rash, and vomiting. In neonates and infants under 3 months of age, additional side effects that were reported irrespective of causality were convulsions, hyperbilirubinemia, and vomiting.
Adults:
Anemia, constipation, diarrhea, headache, nausea and vomiting, and rash are most common. Apnea, injection site reactions (e.g., edema, inflammation, pain, phlebitis), pruritus, sepsis, and shock also occurred in more than 1% of patients. Many other side effects—including CDAD; hypersensitivity reactions (including anaphylaxis); increases or decreases in hematologic, hepatic, and renal lab tests; neuromotor impairment; seizures; and thrombocytopenia—may occur in fewer than 1% of patients.
Post-marketing:
Agranulocytosis, angioedema, erythema multiforme, hemolytic anemia, leukopenia, neutropenia, positive direct or indirect Coombs’ test, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported.
Antidote
Notify physician of all side effects. Most treated symptomatically. Discontinue immediately if a hypersensitivity reaction occurs. Treat hypersensitivity reactions as indicated; may require epinephrine, airway management, oxygen, IV fluids, antihistamines (e.g., diphenhydramine [Benadryl]), corticosteroids (e.g., hydrocortisone sodium succinate [Solu-Cortef]), and pressor amines (e.g., dopamine). If focal tremors, myoclonus, or seizures occur, evaluate neurologically, initiate anticonvulsant therapy, and decide whether to decrease or discontinue meropenem. Mild cases of CDAD may respond to discontinuation of meropenem. Treat CDAD with fluids, electrolytes, protein supplements, and oral vancomycin (Vancocin) or metronidazole (Flagyl) as indicated. In severe cases, surgical evaluation may be indicated. Readily removed by hemodialysis.
Mesna
(MEZ-nah)
Ifosfamide/Mesna Kit, Mesnex, Uromitexan 
Antidote
Antineoplastic adjunct
Prophylactic for hemorrhagic cystitis
pH 6.5 to 8.5
Usual dose
Specific testing recommended before each dose of ifosfamide; see Monitor.
Intravenous dose:
Total daily dose is 60% of the ifosfamide dose equally divided into 3 doses. A single dose of mesna equal to 20% of the ifosfamide dose is given at the time of the ifosfamide injection and repeated 4 hours and 8 hours later (e.g., ifosfamide 1.2 Gm/M2 would require mesna 240 mg/M2 with the ifosfamide, 240 mg/M2 in 4 hours, and again at 8 hours). The initial mesna dose each day may be mixed with the ifosfamide. Appears to be compatible. Available combined in solution with ifosfamide and as a single agent in tablet form.
Combination of intravenous and oral doses:
At the time of the ifosfamide injection, give a single IV dose of mesna equal to 20% of the ifosfamide dose. At 2 hours and at 6 hours after each dose of ifosfamide, administer mesna tablets PO in a dose equal to 40% of the ifosfamide dose. The total daily dose of mesna (IV [20%] and PO [80%] combined) is 100% of the ifosfamide dose.
Dose adjustments
Dose of mesna must be repeated each day ifosfamide is administered and adjusted with each increase or decrease of the ifosfamide dose.
Dilution
Each 100 mg (1 mL) must be diluted in a minimum of 4 mL D5W, D5NS, D5/1/4NS, D5/1/3NS, D5/1/2NS, NS, or LR. Desired concentration is 20 mg/mL.
Filters:
Manufacturer’s studies measured the potency of ifosfamide in combination with mesna through a 5-micron filter. No significant drug loss for ifosfamide; mesna was not measured.
Storage:
Store at CRT before use. Opened multidose vials may be stored at CRT and used for up to 8 days. Diluted solutions are stable for 24 hours at 25° C (77° F). Mesna oxidizes to disulfide dimesna when exposed to oxygen.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
One source suggests the following compatibilities:
Additive:
Cyclophosphamide (Cytoxan), ifosfamide (Ifex).
Y-site:
Allopurinol (Aloprim), amifostine (Ethyol), aztreonam (Azactam), cladribine (Leustatin), docetaxel (Taxotere), doxorubicin liposomal (Doxil), etoposide phosphate (Etopophos), filgrastim (Neupogen), fludarabine (Fludara), gallium nitrate (Ganite), gemcitabine (Gemzar), granisetron (Kytril), linezolid (Zyvox), melphalan (Alkeran), methotrexate, micafungin (Mycamine), ondansetron (Zofran), oxaliplatin (Eloxatin), paclitaxel (Taxol), pemetrexed (Alimta), piperacillin/tazobactam (Zosyn), sargramostim (Leukine), sodium bicarbonate, teniposide (Vumon), thiotepa, vinorelbine (Navelbine).
Rate of administration
A single dose over a minimum of 1 minute given as a single agent. Administer at rate for ifosfamide if given together. Another source recommends administering as an infusion over 15 to 30 minutes or as a continuous infusion maintained for 12 to 24 hours after completion of the ifosfamide infusion.
Actions
A detoxifying agent. Reacts chemically in the kidney with urotoxic ifosfamide metabolites to detoxify them and inhibit hemorrhagic cystitis. Remains in the intravascular compartment and much of a single dose is excreted within 4 hours in urine. Does not appear to interfere with the antitumor efficacy of ifosfamide.
Indications and uses
A prophylactic agent used to reduce the incidence of hemorrhagic cystitis caused by ifosfamide.
Unlabeled uses:
May reduce the incidence of hemorrhagic cystitis caused by cyclophosphamide.
Contraindications
Hypersensitivity to mesna or other thiol compounds.
Precautions
Repeated doses are required to maintain adequate levels of mesna in the kidneys and bladder to detoxify urotoxic ifosfamide metabolites. ■ Hemorrhagic cystitis caused by ifosfamide is dose dependent. Mesna is most effective when ifosfamide dose is less than 1.2 Gm/M2/24 hr. Somewhat less effective when ifosfamide dose is 2 to 4 Gm/M2/24 hr. If hematuria develops with appropriate doses of mesna, ifosfamide dose may need to be reduced or discontinued. ■ Does not inhibit any other side effects or toxicities caused by ifosfamide therapy. ■ Not effective in preventing hematuria caused by other conditions (e.g., thrombocytopenia). ■ Hypersensitivity reactions ranging from mild to anaphylaxis have been reported. Patients with autoimmune disorders who are treated with cyclophosphamide and mesna may have a higher incidence of hypersensitivity reactions.
Monitor:
Before administering each dose of ifosfamide, obtain a morning specimen of urine and test for hematuria. Depending on the severity of the hematuria, dose reduction or discontinuation of ifosfamide may be required. ■ If emesis occurs within 2 hours of taking PO mesna, either repeat the PO dose or administer an IV dose.
Patient education:
Drink at least one quart of liquid daily. ■ Report pink or red urine immediately. ■ Report emesis within 2 hours of taking PO mesna.
Maternal/child:
Category B: use during pregnancy only if benefits clearly outweigh risks. ■ Discontinue breast-feeding. ■ Multidose vial contains benzyl alcohol. Do not use in neonates or infants.
Elderly:
Dosing should be cautious; however, the ratio of ifosfamide to mesna should remain the same.
Drug/lab interactions
May cause a false-positive reaction for urinary ketones. If a red-violet color develops, glacial acetic acid returns the coloring to violet.
Side effects
Average dose:
Anorexia, bad taste in the mouth, coughing, decreased platelets associated with hypersensitivity reactions, diarrhea, dizziness, fever, flushing, headache, hyperesthesia, hypersensitivity reactions, hypertension, hypotension, increased liver enzymes, influenza-like symptoms, injection site reactions, malaise, myalgia, nausea, pharyngitis, soft stool, somnolence, ST-segment elevation, tachycardia, tachypnea, vomiting.
Overdose:
Convulsions, cyanosis, diarrhea, dyspnea, fatigue, headache, hematuria, hypersensitivity reactions, hypotension, limb pain, nausea, tremor.
Antidote
No specific antidote. Keep physician informed of all side effects. Notify promptly if signs of overdose occur. Resuscitate as necessary.
Methadone hydrochloride 
(METH-ah-dohn hy-droh-KLOR-eyed)
Dolophine
Opioid analgesic (agonist)
Narcotic abstinence syndrome suppressant
pH 4.5 to 6.5
Usual dose
Parenteral administration permitted only under specific conditions; see Indications and Precautions.
Dosing is complex and requires extensive individualization. Extended half-life, potential for prolonged respiratory depressant effects, retention in the liver (prolonging the potential duration of action), and high interpatient variability in absorption, metabolism, and relative analgesic potency must be considered.
Treatment of pain:
Consider the following factors for each patient before determining an initial dose:
• Total daily dose, potency, and specific characteristics of any previously administered opioid.
• Will it be used for acute or chronic methadone dosing?
• Patient’s degree of opioid tolerance.
• Patient’s age, general condition, and medical status.
• Concurrent medications; see Drug/Lab Interactions.
• Type, severity, and expected duration of patient’s pain.
• Acceptable balance between pain control and adverse effects.
Initial analgesic in patients who are not being treated with other opioids and are not tolerant to other opioids:
2.5 to 10 mg every 8 to 12 hours. Titrate slowly to effect. More frequent dosing may be required to maintain adequate analgesia; use extreme caution, consider extended half-life, and avoid overdose.
Conversion from oral to parenteral methadone:
Begin with a 2:1 ratio (e.g., 10 mg oral methadone to 5 mg parenteral methadone); see Precautions.
Switching from other chronic opioids to parenteral methadone:
Dose conversion ratios and cross-tolerance are uncertain; deaths have occurred in opioid-tolerant patients during conversion to methadone. Individualize dose based on patient’s prior opioid exposure, general medical condition, concomitant medication, and anticipated breakthrough medication use. Titrate to achieve adequate pain relief balanced against tolerability of side effects. Adjust dose and/or dosing interval as necessary. The following two charts are examples of conversions.
| Conversion from Oral Morphine to IV Methadone for Chronic Administration | ||
| Total Daily Baseline Oral Morphine Dose | Estimated Total Daily Oral Methadone Dose as a Percentage of Total Daily Morphine Dose* | Estimated Daily IV Methadone Dose as a Percentage of Total Daily Oral Morphine Dose* |
| <100 mg | 20% to 30% | 10% to 15% |
| 100 mg to 300 mg | 10% to 20% | 5% to 10% |
| 300 mg to 600 mg | 8% to 12% | 4% to 6% |
| 600 mg to 1,000 mg | 5% to 10% | 3% to 5% |
| >1,000 mg | Less than 5% | Less than 3% |
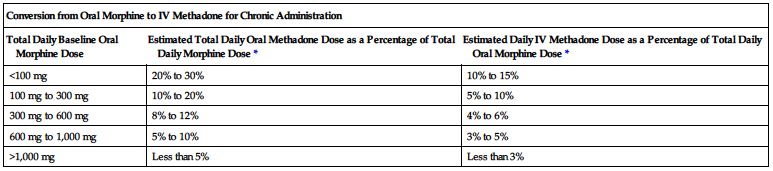
*Divide total daily methadone dose as necessary to achieve desired dosing schedule (e.g., divide by 3 for administration every 8 hours).
| Conversion from Parenteral Morphine to IV Methadone for Chronic Administration | |
| Total Daily Baseline Parenteral Morphine Dose | Estimated Daily IV Methadone Dose as a Percentage of Total Daily Morphine Dose* |
| 10 mg to 30 mg | 40% to 66% |
| 30 mg to 50 mg | 27% to 66% |
| 50 mg to 100 mg | 22% to 50% |
| 100 mg to 200 mg | 15% to 34% |
| 200 mg to 500 mg | 10% to 20% |
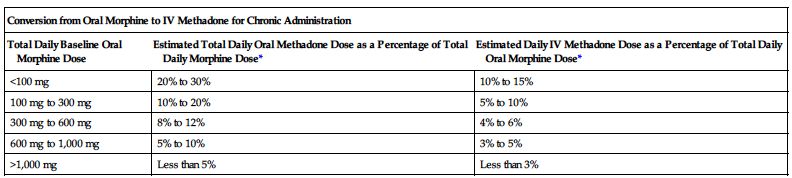
*Divide total daily methadone dose as necessary to achieve desired dosing schedule (e.g., divide by 3 for administration every 8 hours).
Dose adjustments
Lower-end initial doses are indicated in the elderly; consider impaired organ function and concomitant disease or drug therapy. ■ Reduced initial doses are indicated in debilitated patients and in those with severe impaired hepatic or renal function. ■ Clearance may be increased and half-life decreased during pregnancy. Increased doses or shorter intervals between doses may be indicated; see Maternal/Child.
Dilution
May be given undiluted; however, to facilitate titration, further dilution of each mL with 1 to 5 mL of NS is appropriate. May be given through Y-tube or three-way stopcock of infusion set.
Storage:
A multidose vial; store in carton at CRT protected from light until contents have been used.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
One source suggests the following compatibilities:
Y-site:
Atropine, dexamethasone (Decadron), diazepam (Valium), diphenhydramine (Benadryl), ketorolac (Toradol), lorazepam (Ativan), metoclopramide (Reglan), midazolam (Versed), phenobarbital (Luminal).
Rate of administration
A single dose as an injection over a minimum of several minutes. Titrate according to symptom relief and respiratory rate; side effects may be increased if rate of injection is too rapid.
Actions
A synthetic opioid analgesic with actions similar to those of morphine. Duration of analgesic action is from 4 to 8 hours. Respiratory depressant effects occur later and persist longer than its peak analgesic effects. With repeated dosing, methadone may be retained in the liver and then slowly released, thereby prolonging the duration of action while plasma concentrations are low. Highly protein bound (85% to 90%). Metabolized in the liver by various enzymes (including cytochrome P450 enzymes) to inactive metabolites. Half-life is prolonged (ranges from 8 to 59 hours). Eliminated by extensive biotransformation followed by renal and fecal elimination. Secreted in saliva, breast milk, amniotic fluid, and umbilical cord plasma.
Indications and uses
Treatment of moderate to severe pain not responsive to nonnarcotic analgesics. ■ Temporary treatment in opioid-dependent patients unable to take oral medication. ■ Used PO for detoxification or maintenance in opioid addiction. ■ Parenteral products are not approved for outpatient use.
Contraindications
Known hypersensitivity to methadone or any of its components. ■ Any situation in which opioids are contraindicated (e.g., patients with respiratory depression [where resuscitative equipment is not readily available or in unmonitored settings], patients with acute bronchial asthma or hypercarbia). ■ Other sources add paralytic ileus and concurrent use of selegiline (Zelapar, Eldepryl).
Precautions
If the parenteral route is indicated, the IV route is preferred; absorption by the IM or SC routes is unpredictable and may cause local tissue reaction. ■ Schedule II opioid agonists, including hydromorphone, morphine, oxymorphone, oxycodone, fentanyl, and methadone, have the highest potential for abuse and risk of producing respiratory depression. Alcohol, CNS depressants, and other opioids potentiate the respiratory depressant effects of hydromorphone, increasing the risk of respiratory depression that might result in death; see Drug/Lab Interactions. ■ Deaths, cardiac and respiratory, have been reported during initiation and conversion of pain patients to methadone treatment from treatment with other opioid agonists. Close monitoring is required during these times as well as during dose titration. ■ The peak respiratory depressant effects of methadone usually occur later and last longer than the peak analgesic effects. Iatrogenic overdose may occur with short-term use, particularly during treatment initiation and dose titration. ■ Patients tolerant to other opioids may still be sensitive to methadone. This incomplete cross-tolerance makes dose selection difficult when converting to methadone. Deaths have been reported. A high degree of “opioid tolerance” does not eliminate the possibility of methadone toxicity. ■ Prolongation of the QT interval and infrequent cases of arrhythmia (including torsades de pointes) have been reported. May occur with any dose but appears to be more common with higher dose treatment (greater than 200 mg/day). ■ Use with caution in patients at risk for development of prolonged QT interval (e.g., cardiac conduction abnormalities, cardiac hypertrophy, hypokalemia, hypomagnesemia) and in patients receiving concurrent treatment with other drugs that may induce electrolyte disturbances (e.g., diuretics, laxatives and, rarely, mineralocorticoid hormones) or other drugs that may prolong the QT interval; see Drug/Lab Interactions. ■ Initiate treatment for analgesic therapy in patients with acute or chronic pain only if benefits outweigh the increased risk of QT prolongation with high doses. ■ Use extreme caution in patients with potential respiratory insufficiency (e.g., elderly or debilitated patients, conditions accompanied by hypoxia or hypercapnia or decreased respiratory reserve [e.g., asthma, COPD, pulmonary disease or cor pulmonale, severe obesity, sleep apnea syndrome, myxedema, kyphoscoliosis, CNS depression, or coma]). Usual doses may decrease respiratory drive and simultaneously increase airway resistance, resulting in apnea. Consider use of non-opioid analgesics. If methadone is required, administer at lowest effective dose under close medical supervision. ■ Use caution in elderly or debilitated patients, severe impaired hepatic or renal function, Addison’s disease, hypothyroidism, prostatic hypertrophy, or urethral stricture; see Dose Adjustments. ■ Use with caution in patients with head injury, other intracranial lesions, or pre-existing increased intracranial pressure. Cerebrospinal fluid pressure may be markedly exaggerated, and the clinical course of head injuries may be obscured. ■ May mask symptoms and make diagnosis of acute abdominal conditions difficult. ■ May cause severe hypotension in patients whose ability to maintain normal blood pressure is compromised (e.g., severe volume depletion). ■ Patients with impaired hepatic function may be at increased risk of accumulating methadone after multiple dosing. ■ Because of their opioid tolerance, patients receiving maintenance doses may require increased or more frequent doses of opioids to treat physical trauma, postoperative pain, or other acute pain. ■ Do not increase methadone dose for symptoms of anxiety. ■ Abrupt discontinuation of methadone is not recommended; may result in withdrawal symptoms (e.g., chills, lacrimation, myalgia, mydriasis, perspiration, restlessness, rhinorrhea, yawning) and may lead to relapse of illicit drug use.
Monitor:
Oxygen, controlled ventilation equipment, and naloxone must always be available. ■ Observe patient frequently to continuously based on amount of dose, and monitor VS. ■ Assess baseline pain, then assess pain with vital signs or more frequently if needed. Reassess after administration of methadone and adjust dose or interval as required. Keep patient supine; orthostatic hypotension and fainting may occur; monitor closely during ambulation. ■ Uncontrolled pain causes sleep deprivation, decreases pain threshold, and increases pain. When pain is finally controlled, expect the patient to sleep more until recovered from sleep deprivation. ■ Monitor ECG in patients who develop QT prolongation and assess for modifiable risk factors (e.g., drugs with cardiac effects, drugs that may cause electrolyte abnormalities, or drugs that may inhibit the metabolism of methadone); see Drug/Lab Interactions. Consider alternate therapies for pain management. ■ Laxatives with or without stool softeners will be required to avoid constipation and fecal impaction, especially with increased doses and extended use. Maintain adequate hydration. ■ See Precautions and Drug/Lab Interactions.
Patient education:
Promptly report dizziness, light-headedness, palpitations, or syncope; may indicate a need for ECG monitoring. ■ Avoid alcohol or other CNS depressants (e.g., barbiturates, benzodiazepines [e.g., diazepam (Valium)]). ■ May cause blurred vision, dizziness, or drowsiness; use caution in tasks that require alertness. ■ Request assistance with ambulation. ■ May be habit forming.
Maternal/child:
Category C: potential benefit should justify potential risk to fetus. Compare the benefit of methadone to the risk of untreated addiction to illicit drugs. ■ Total body clearance is increased during pregnancy and half-life is decreased (during 2nd and 3rd trimesters). May lead to withdrawal symptoms. Increases in dose or dosing at more frequent intervals may be indicated. ■ Data is insufficient; however, women treated with methadone during pregnancy had improved prenatal care and did not appear to have an increased risk of miscarriage or premature delivery. Methadone in amniotic fluid and cord plasma has similar concentrations to maternal plasma. Newborn urine concentrations are less than maternal concentrations. ■ Infants born to women treated with methadone during pregnancy may experience respiratory depression (consider methadone’s long duration of action) and may be born physically dependent. Onset of withdrawal may occur in days or be delayed for 2 to 4 weeks. Withdrawal signs include fever, hyperactive reflexes, increased respiratory rate, increased stools, irritability and excessive crying, sneezing, tremors, vomiting, and yawning. May have reduced birth weight, length, and/or head circumference and some deficits in psychometric and behavioral tests. ■ Discontinue or do not start breast-feeding. Women who are breast-feeding should wean gradually to prevent withdrawal symptoms in their infants. ■ Safety and effectiveness for use in pediatric patients under 18 years of age not established. ■ See Drug/Lab Interactions.
Elderly:
Dosing should be cautious; see Dose Adjustments. ■ Differences in response compared to younger adults not identified. ■ See Precautions.
Drug/lab interactions
May have additive effects with other CNS depressants (e.g., alcohol, general anesthetics, hypnotics or sedatives [e.g., benzodiazepines (e.g., diazepam [Valium], midazolam [Versed]), barbiturates [e.g., phenobarbital (Luminal)], other opioid analgesics, phenothiazines [e.g., prochlorperazine (Compazine)]). Concurrent use may result in hypotension, profound sedation, respiratory depression, coma, or death. ■ Not recommended for concurrent administration with opioid antagonists (e.g., naloxone, naltrexone [ReVia]), mixed agonist/antagonists, or partial agonists (e.g., buprenorphine [Buprenex], butorphanol [Stadol], nalbuphine, pentazocine [Talwin]); may precipitate withdrawal symptoms and/or reduce analgesic effect in patients maintained on methadone. ■ Metabolism increased and serum concentrations decreased by cytochrome P450 inducers (e.g., carbamazepine [Tegretol], phenobarbital [Luminal], phenytoin [Dilantin], rifampin [Rifadin], St. John’s wort), efavirenz (Sustiva), nevirapine (Viramune), ritonavir (Norvir), and ritonavir/lopinavir combination (Kaletra). With concurrent administration, the effects of methadone are decreased; monitor for S/S of withdrawal, and adjust methadone dose as indicated. ■ Metabolism decreased and serum concentrations increased by cytochrome P450 inhibitors (e.g., azole antifungal agents [e.g., itraconazole (Sporanox), ketoconazole (Nizoral)], macrolide antibiotics [e.g., erythromycin], selective serotonin reuptake inhibitors (SSRIs) [e.g., fluvoxamine (Luvox), sertraline (Zoloft)]). With concurrent administration, monitor methadone serum concentrations to prevent methadone toxicity. ■ May increase serum levels of desipramine (Norpramin). ■ May increase AUC of zidovudine and result in zidovudine toxicity. ■ May decrease AUC and peak levels of didanosine (Videx) and stavudine (Zerit). ■ Use caution with MAO inhibitors (e.g., selegiline [Eldepryl]) administered within 14 days and test for sensitivity. MAO inhibitors have caused cardiovascular collapse with other opioids (e.g., meperidine [Demerol]), not reported with methadone; see Contraindications. ■ Use extreme caution with other drugs that prolong the QT interval (e.g., Class Ia antiarrhythmic agents [e.g., quinidine, procainamide (Pronestyl)], Class III antiarrhythmic agents [e.g., amiodarone (Nexterone), sotalol (Betapace)], calcium channel blockers [e.g., diltiazem (Cardizem), verapamil], some neuroleptics [e.g., phenothiazines (e.g., chlorpromazine [Thorazine])], and tricyclic antidepressants [e.g., amitriptyline (Elavil), imipramine (Tofranil)]).
Side effects
Hypotension and respiratory depression are dose limiting. Respiratory arrest, shock, cardiac arrest, and death have occurred.
Dizziness, light-headedness, nausea and vomiting, sedation, and sweating occur most frequently. Other reported side effects include abdominal pain, agitation, anorexia, antidiuretic effect, arrhythmias, asthenia, biliary tract spasm, cardiomyopathy, confusion, constipation, chronic hepatitis, dry mouth, edema, euphoria, flushing, glossitis, headache, heart failure, hypokalemia, hypomagnesemia, hypotension, palpitations, phlebitis, prolonged QT interval, pulmonary edema, pruritus, seizures, skin rashes, syncope, thrombocytopenia (reversible), torsades de pointes, urinary retention or hesitancy, urticaria, visual disturbances.
Overdose:
Bradycardia, cold and clammy skin, constricted pupils, hypotension, respiratory depression (decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), skeletal muscle flaccidity, somnolence progressing to stupor or coma. Apnea, circulatory collapse, cardiac arrest, and death may occur.
Antidote
Keep physician informed of all side effects; may be treated symptomatically. During prolonged administration (patients on maintenance), side effects usually decrease with time. Lower doses of methadone may be indicated for dizziness, light-headedness, nausea and vomiting, sedation, and sweating. Management of major side effects or overdose requires establishing a patent airway, instituting assisted or controlled ventilation, IV fluids, vasopressors, and other supportive measures as indicated. Do not administer opioid antagonists to physically dependent patients unless clinically significant respiratory or cardiovascular depression occurs. May precipitate an acute withdrawal syndrome. If a decision is made to treat serious respiratory or circulatory depression in a physically dependent patient, administration of the antagonist should begin with care and by titration with smaller than usual doses of the antagonist. Opioid antagonists (e.g., naloxone)have a much shorter duration of action than methadone and may need repeating for up to 48 hours. Continuous monitoring is required. Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion may not be useful to increase methadone or metabolite elimination; however, urine acidification has been shown to increase renal elimination of methadone.
Methotrexate sodium 
(meth-oh-TREKS-ayt SO-dee-um)
Methotrexate PF, MTX
Antineoplastic (antimetabolite)
Antipsoriatic
Antirheumatic
pH 8.5
Usual dose
Many dose limitations based on patient condition, renal and hepatic function, and concomitant drugs or therapies; see Precautions/Monitor. Doses between 100 and 500 mg/M2 may require leucovorin calcium rescue. Doses over 500 mg/M2 require leucovorin calcium rescue; see leucovorin calcium or levoleucovorin (Fusilev) monograph. Part of numerous protocols that change as new advances in antileukemic therapy and other cancers are developed. Selections from those protocols are included in the following text.
Acute lymphoblastic leukemia:
Induction:
3.3 mg/M2 in combination with prednisone 60 mg/M2. Give daily if tolerated, and continue for up to 8 weeks or until satisfactory response (usually 4 to 6 weeks). Usually given PO.
Maintenance:
Dose individualized; 15 mg/M2/dose administered 2 times weekly IM or PO (a total weekly dose of 30 mg/M2) or 2.5 mg/kg IV every 14 days has been used.
Mycosis fungoides:
5 to 50 mg once weekly in the early stages of disease. Adjust dose or discontinue as indicated by patient response and hematologic monitoring. 15 to 37.5 mg twice weekly may be used in patients who respond poorly to weekly therapy. Usually given PO or IM, but combination chemotherapy regimens, including higher doses of IV methotrexate with leucovorin calcium rescue, have been used in advanced stages of the disease.
Breast cancer (unlabeled):
One regimen administers methotrexate 40 mg/M2 on Days 1 and 8 of each cycle. Given in combination with PO cyclophosphamide 100 mg/M2 on Days 1 through 14 of each cycle and fluorouracil 600 mg/M2 on Days 1 and 8 of each cycle. In patients over 60 years of age, reduce the initial methotrexate dose to 30 mg/M2 and the initial fluorouracil dose to 400 mg/M2. Repeat monthly (allows a 2-week rest period between cycles) for 6 to 12 cycles.
Psoriasis:
10 to 25 mg once a week until adequate response. Some references suggest an initial test dose of 5 to 10 mg/week before initiating therapy to detect sensitivity to adverse reactions. Sources suggest 30 mg/week as a maximum dose. Use smallest effective dose. Usually given PO or IM. May be used in combination with infliximab; see infliximab monograph.
Osteosarcoma:
One regimen recommends 12 Gm/M2 as a single dose given as an infusion over 4 hours. Begin the fourth week after surgery and repeat weekly at Weeks 5, 6, 7, 11, 12, 15, 16, 29, 30, 44, and 45. A peak serum concentration of 1,000 micromolars/L at the end of the infusion is desired. Dose may be increased to 15 Gm/M2 if required. Must be accompanied by leucovorin calcium rescue; see leucovorin calcium or levoleucovorin (Fusilev) monograph. Leucovorin calcium may be given IV or PO; levoleucovorin is IV only. When methotrexate is given in combination with leucovorin rescue, the serum creatinine must be normal, and creatinine clearance must be greater than 60 mL/min before beginning therapy. Osteosarcoma also requires combination chemotherapy. Protocols vary but may include methotrexate in combination with doxorubicin, with cisplatin, and with the combination of bleomycin, cyclophosphamide, and dactinomycin (BCD regimen). These massive doses are highly individualized and require exacting calculations and constant patient monitoring; see Precautions/Monitor.
Pediatric dose
Safety for use in pediatric patients is limited to chemotherapy and in polyarticular-course juvenile rheumatoid arthritis. May contain benzyl alcohol; not recommended for use in neonates. See Maternal/Child.
Dose adjustments
Manufacturer does not provide information on dose adjustment in patients with impaired renal or hepatic function. However, various recommendations are available in the literature. In patients with impaired hepatic function, one source recommends administering 75% of dose if bilirubin is between 3.1 and 5 or if transaminases are greater than 3 times the ULN; if bilirubin above 5, omit dose. ■ Reduced doses may be required in patients with impaired renal function. Suggested guidelines are to administer 50% of a dose with a CrCl of 10 to 50 mL/min, and avoid use with a CrCl of less than 10 mL/min in adult patients. In pediatric patients, administer 50% of a dose with a CrCl of 10 to 50 mL/min and 30% of a dose with a CrCl of less than 10 mL/min/1.73M2. ■ Reduced dose may be required in patients with ascites or pleural effusions, in the very young or very elderly, in the debilitated, and in other diseases; see Precautions. ■ Often used with other antineoplastic drugs to achieve tumor remission. ■ See Drug/Lab Interactions.
Dilution
Specific techniques required; see precautions.
Available in solution or as a lyophilized powder. Reconstitute powder with D5W or NS. The 1-Gm vial should be reconstituted with 19.4 mL to a concentration of 50 mg/mL. When high-dose methotrexate is administered by IV infusion, the total dose is diluted in D5W. 25 mg/mL is the maximum suggested concentration that can be given IV. Reconstitution of each 5 mg with 2 mL of preservative-free D5W or NS is suggested. Each milliliter equals 2.5 mg of methotrexate. Available in preservative-free solution. Do not use formulations or diluents with preservatives (e.g., bacteriostatic) for high-dose therapy or intrathecal injection. 1-Gm vial available for high-dose use with appropriate dilution. Not usually added to IV solutions when given in smaller doses (less than 100 mg). Discard solution if a precipitate forms. May be given through Y-tube or three-way stopcock of a free-flowing IV.
A single dose may be further diluted with D5W or NS immediately before use as an infusion with higher (100 mg or more) methotrexate doses.
Filters:
No data available from manufacturer. Another source indicates no significant drug loss filtered through a nylon 0.2-micron filter.
Storage:
Store in unopened container at CRT; protect from light. If prepared without a preservative, use immediately. May be stable up to 24 hours with a preservative.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
One source suggests the following compatibilities:
Additive:
Cyclophosphamide (Cytoxan), cytarabine (ARA-C), fluorouracil (5-FU), ondansetron (Zofran), sodium bicarbonate, vincristine. Other sources add dacarbazine (DTIC), furosemide (Lasix), hydrocortisone sodium succinate (Solu-Cortef), leucovorin calcium.
Y-site:
Allopurinol (Aloprim), amifostine (Ethyol), amphotericin B cholesteryl (Amphotec), asparaginase (Elspar), aztreonam (Azactam), bleomycin (Blenoxane), ceftriaxone (Rocephin), cisplatin, cyclophosphamide (Cytoxan), cytarabine (ARA-C), daunorubicin (Cerubidine), doripenem (Doribax), doxorubicin (Adriamycin), doxorubicin liposomal (Doxil), etoposide (VePesid), etoposide phosphate (Etopophos), filgrastim (Neupogen), fludarabine (Fludara), fluorouracil (5-FU), furosemide (Lasix), gallium nitrate (Ganite), granisetron (Kytril), heparin, imipenem-cilastatin (Primaxin), leucovorin calcium, linezolid (Zyvox), melphalan (Alkeran), mesna (Mesnex), methylprednisolone (Solu-Medrol), metoclopramide (Reglan), mitomycin (Mutamycin), ondansetron (Zofran), oxacillin (Bactocill), oxaliplatin (Eloxatin), paclitaxel (Taxol), piperacillin/tazobactam (Zosyn), sargramostim (Leukine), teniposide (Vumon), thiotepa, vancomycin, vinblastine, vincristine, vinorelbine (Navelbine).
Rate of administration
IV injection:
Each 10 mg or fraction thereof over 1 minute.
Infusion:
A single dose equally distributed over 30 minutes to 4 hours or as prescribed by protocol.
Actions
An antimetabolite and folic acid antagonist. Inhibits dihydrofolic acid reductase. Cell cycle–specific for the S phase. It interferes with DNA synthesis, repair, and cellular replication. Rapidly proliferating tissues are more sensitive to this effect. Widely distributed and is approximately 50% protein bound. Undergoes some hepatic and intracellular metabolism. Half-life is dose dependent and is 3 to 10 hours in patients receiving low-dose antineoplastic therapy and 8 to 15 hours in patients receiving high-dose methotrexate therapy. 80% to 90% of the administered dose is excreted unchanged in the urine within 24 hours. Clearance rates decrease with higher doses. Does not cross blood-brain barrier. Secreted in breast milk.
Indications and uses
Used for life-threatening neoplastic disease alone or in combination with other anticancer agents in the treatment of acute lymphocytic leukemia, breast cancer, epidermal tumors of the head and neck, small-cell and squamous cell lung cancer, non-Hodgkin’s lymphoma, advanced mycosis fungoides (cutaneous T-cell lymphoma). ■ Severe disabling psoriasis or rheumatoid arthritis unresponsive to other treatment. Diagnosis of psoriasis should be established by biopsy and/or dermatology consultation before use. ■ To prolong relapse-free survival in patients with nonmetastatic osteosarcoma who have undergone surgical resection or amputation of the primary tumor. Given as a high-dose regimen with leucovorin rescue in combination with other chemotherapeutic agents. ■ Given PO or IM for early-stage mycosis fungoides, trophoblastic diseases (gestation choriocarcinoma, chorioadenoma destruens, and hydatidiform mole), polyarticular-course juvenile rheumatoid arthritis, rheumatoid arthritis, and other diagnoses, and given intrathecally for treatment and prophylaxis of meningeal leukemia.
Unlabeled uses:
Treatment of bladder and testicular cancer. Treatment of soft tissue sarcomas and CNS tumors. Management of Crohn’s disease and ectopic pregnancy and prevention of acute graft-versus-host disease. High-dose regimens for neoplastic diseases other than osteosarcoma are investigational, and therapeutic efficacy is not established.
Contraindications
Hypersensitivity to methotrexate; breast-feeding mothers. Contraindicated in pregnant females with psoriasis or rheumatoid arthritis and should be used during pregnancy for treatment of neoplastic diseases only when the potential benefit outweighs the risk to the fetus. Contraindicated in psoriasis or rheumatoid arthritis patients with immunodeficiency syndromes, pre-existing blood dyscrasias, or chronic liver disease.
Precautions
Follow guidelines for handling cytotoxic agents. See Appendix A, p. 1331. ■ Administered by or under the direction of the physician specialist. ■ Methotrexate should be used only in life-threatening neoplastic diseases or in patients with psoriasis or rheumatoid arthritis with severe, recalcitrant, disabling disease that is not responsive to other forms of therapy. ■ Deaths have been reported with methotrexate use in the treatment of malignancy, psoriasis, and rheumatoid arthritis. ■ Methotrexate elimination is reduced in patients with impaired renal function, ascites, or pleural effusions. Careful monitoring, dose reduction and, in some cases, discontinuation of therapy are required; consider evacuating excess fluid from ascites and pleural effusions before treatment if possible. ■ Methotrexate can suppress hematopoiesis, causing anemia, aplastic anemia, pancytopenia, leukopenia, neutropenia, and/or thrombocytopenia. ■ Serious and sometimes fatal bone marrow suppression, aplastic anemia, and GI toxicity have been reported with concomitant use of methotrexate (usually high doses) and some NSAIDs; see Drug/Lab Interactions. ■ Leukoencephalopathy has been reported in patients who have received both IV methotrexate and craniospinal irradiation. ■ A transient stroke-like encephalopathy has been reported with high-dose methotrexate therapy. Symptoms may include confusion, hemiparesis, transient blindness, seizures, and coma. ■ Methotrexate-induced lung disease, including acute or chronic interstitial pneumonitis, may occur at any time, may occur even with low doses, and has been fatal. Patients may present with fever, dry cough, dyspnea, hypoxemia, and an infiltrate on chest x-ray. May require interruption of therapy and is not always fully reversible. ■ Severe skin reactions can occur at any time and have been fatal. ■ Transient elevations in liver enzymes may occur early during treatment. Chronic hepatotoxicity (fibrosis and cirrhosis) occurs more frequently with prolonged use and a total dose of at least 1.5 Gm. Persistent abnormalities in liver function tests and/or depression of serum albumin may be indicators of serious liver toxicity. Has resulted in deaths; see Monitor. ■ Use with extreme caution in patients with ascites, bone marrow suppression, folate deficiency, GI obstruction, impaired renal or liver function, infection, peptic ulcer, pleural effusion, or ulcerative colitis; in debilitated patients; and in the very young or very elderly. ■ Diarrhea and ulcerative stomatitis will require interrupting therapy; otherwise hemorrhagic enteritis and death from intestinal perforation may occur. ■ Tumor lysis syndrome may occur, and S/S include hyperkalemia, hyperphosphatemia, hyperuricemia, hypocalcemia, metabolic acidosis, urate crystalluria, and renal failure. Prevent or alleviate tumor lysis syndrome with appropriate supportive and pharmacologic measures; see Monitor. ■ Potentially fatal opportunistic infections, especially Pneumocystis jiroveci pneumonia, may occur. ■ Risk of soft tissue necrosis and osteonecrosis may be increased with concomitant use of methotrexate and radiotherapy. ■ May cause renal damage that may lead to acute renal failure. Nonreversible oliguric renal failure is likely to develop in patients who experience delayed early methotrexate elimination. ■ Malignant lymphomas have been reported. May occur in patients receiving low-dose methotrexate and may not require cytotoxic treatment; discontinue methotrexate first and initiate appropriate treatment if the lymphoma does not regress. ■ Use caution when administering high-dose methotrexate to patients receiving proton pump inhibitors; see Drug Interactions.
Monitor:
Close patient observation is mandatory. Course of therapy is not repeated until all signs of toxicity from the previous course subside. ■ CBC with platelets, chest x-ray, and renal and liver function tests before, during, and after therapy are essential to comprehensive treatment. ■ Monitor closely for bone marrow, liver, lung, kidney, and skin toxicities. ■ Nadir of leukocyte and platelet count usually occurs after 7 to 10 days, with recovery 7 days later. ■ Liver biopsy, pulmonary studies, and bone marrow studies may be indicated in high-dose or long-term therapy. ■ Monitor renal function closely; verify by CrCl levels; see Precautions. Maintain continuing adequate hydration and urine alkalinization. ■ Prevention and treatment of hyperuricemia due to tumor lysis syndrome may be accomplished with adequate hydration and, if necessary, allopurinol and alkalinization of urine. ■ Monitor serum methotrexate levels. ■ Use prophylactic antiemetics to reduce nausea and vomiting and increase patient comfort. ■ Observe closely for signs of infection. Prophylactic antibiotics may be indicated pending results of C/S in a febrile neutropenic patient. ■ Monitor for thrombocytopenia (platelet count less than 50,000/mm3). Initiate precautions to prevent excessive bleeding (e.g., inspect IV sites, skin, and mucous membranes; use extreme care during invasive procedures; test urine, emesis, stool, and secretions for occult blood). ■ Administration of high-dose methotrexate requires the following: a WBC count greater than 1,500/mm3, neutrophil count greater than 200/mm3, platelet count greater than 75,000/mm3, serum bilirubin less than 1.2 mg/dL, alanine aminotransferase (ALT) level less than 450 units, healing of any mucositis, ascites or pleural effusion must be drained dry, normal SCr, CrCl greater than 60 mL/min, 1 L/M2 of IV fluid over 6 hours before dosing and 3 L/M2/day on day of infusion and for 2 days after, alkalinization of urine with sodium bicarbonate to maintain pH above 7, and repeat serum methotrexate and SCr levels at least daily until methotrexate level is below 0.05 micromolar. ■ See Drug/Lab Interactions.
Patient education:
Avoid pregnancy. Nonhormonal birth control recommended for both females and males. Continue for at least 3 months after treatment is complete in male patients and for at least one ovulatory cycle after therapy is complete for female patients. ■ Avoid alcohol and take only prescribed medications. Reactions can be lethal. ■ Side effects such as dizziness and fatigue may interfere with ability to drive or operate machinery. ■ Review early signs and symptoms of toxicity with health care provider. ■ Close follow-up with physician is imperative. ■ See Appendix D, p. 1333.
Maternal/child:
Category X: avoid pregnancy. Has caused fetal death and congenital anomalies. ■ Discontinue breast-feeding. ■ Safety for use in pediatric patients established for cancer chemotherapy and polyarticular-course juvenile rheumatoid arthritis. ■ Serious neurotoxicity (e.g., general or focal seizures) has been reported in patients with acute lymphoblastic leukemia who have been treated with intermediate-dose methotrexate. ■ Administration of formulations containing the preservative benzyl alcohol have been associated with fatal gasping syndrome in neonates. Use preservative-free formulation of methotrexate in neonates.
Elderly:
See Dose Adjustments. Dose selection should be cautious and based on the potential for decreased organ function, decreased folate stores, and concomitant disease or drug therapy. ■ Consider monitoring CrCl and methotrexate levels. ■ Monitor for early signs of hepatic, renal, or bone marrow toxicity. ■ In chronic administration, certain toxicities may be decreased by folate supplementation. ■ Post-marketing experience suggests that the occurrence of bone marrow suppression, thrombocytopenia, and pneumonitis may increase with age.
Drug/lab interactions
The following drugs may enhance methotrexate toxicity when administered concomitantly: cyclosporine (Sandimmune), any hepatotoxic drug (e.g., azathioprine, retinoids [vitamin A], sulfasalazine [Azulfidine]), etretinate (Tegison), NSAIDs (e.g., ibuprofen [Advil, Motrin], ketoprofen, naproxen [Aleve, Naprosyn]), penicillins (e.g., amoxicillin, nafcillin [Nallpen]), probenecid, salicylates, sulfonamides, phenytoin (Dilantin), pyrimethamine, trimethoprim (component of sulfamethoxazole/trimethoprim), and vancomycin (given up to 10 days prior to methotrexate); interactions may be life threatening. Monitoring serum levels and/or reduced doses of methotrexate may be indicated or a longer duration of leucovorin calcium rescue may be required. One source suggests delaying administration of aspirin, NSAIDs, and probenecid for 48 hours after larger doses of methotrexate; see Precautions. ■ NSAIDs are used in the treatment of rheumatoid arthritis in combination with low doses of methotrexate (e.g., 7.5 to 15 mg/week). Do not administer NSAIDs before or concomitantly with high doses of methotrexate (e.g., treatment of osteosarcoma); deaths from severe hematologic and GI toxicity have been reported. ■ Use caution if high-dose methotrexate is administered in combination with nephrotoxic chemotherapy agents (e.g., cisplatin). ■ Concurrent use of methotrexate (primarily at high dose) with some proton pump inhibitors such as esomeprazole (Nexium), omeprazole (Prilosec), and pantoprazole (Protonix) may cause prolonged elevation of serum levels of methotrexate and its metabolite, hydroxymethotrexate, leading to toxicity. Discontinue several days before methotrexate administration. Consider an H2 antagonist (e.g., ranitidine [Zantac]). ■ Doxycycline may increase toxicity of methotrexate in high-dose regimens. ■ Vitamins with folic acid may inhibit the antifolate effects of methotrexate, decreasing effectiveness. ■ May increase serum levels of mercaptopurine (Purinethol); dose adjustment may be required. ■ Do not administer live virus vaccines to patients receiving antineoplastic drugs. ■ Procarbazine (Matulane) may increase nephrotoxicity of methotrexate. Allow 72 hours between last dose of procarbazine and first dose of methotrexate. ■ Monitor for signs of increased bone marrow suppression with sulfonamides (e.g., sulfisoxazole [Gantrisin], SMZ-TMP [Bactrim]), bone marrow–suppressing agents (e.g., antineoplastics), and radiation therapy. May also cause SMZ-TMP (Bactrim)-induced megaloblastic anemia. ■ May decrease theophylline clearance and increase serum levels; monitor theophylline serum levels with concurrent use. ■ Urinary alkalinizers increase renal excretion and may reduce effectiveness.
Side effects
Toxicity usually dose related. Death can occur from average doses, high doses, drug interactions (e.g., NSAIDs), bone marrow toxicity, GI toxicity, hepatic toxicity, pulmonary toxicity, and/or severe skin reactions. Abdominal distress, chills, decreased resistance to infection, dizziness, fatigue, fever, leukopenia, malaise, nausea, and ulcerative stomatitis occur most frequently. Other side effects reported include abortion (spontaneous), acne, acute hepatitis, agranulocytosis, alopecia, alveolitis, anaphylaxis, anemia, anorexia, aplastic anemia, azotemia, blurred vision, chronic fibrosis and cirrhosis, convulsions, COPD, cystitis, decreased serum albumin, defective oogenesis or spermatogenesis, diabetes, diarrhea, drowsiness, enteritis, eosinophilia, erythema multiforme, erythematous rashes, exfoliative dermatitis, fetal defects or death, furunculosis, GI ulceration and bleeding, gingivitis, gynecomastia, headache, hematemesis, hematuria, hemiparesis, hepatic failure, hepatotoxicity, hypotension, infertility, interstitial pneumonitis, liver enzyme elevations, lymphadenopathy and lymphoproliferative disorders, melena (passage of dark, tarry stools), menstrual dysfunction, nephropathy (severe), neutropenia, oligospermia (transient), opportunistic infections (e.g., cytomegalovirus infection, herpes zoster, histoplasmosis, Pneumocystis jiroveci pneumonia), pancreatitis, pancytopenia, paresis, pericardial effusion, pericarditis, pharyngitis, photosensitivity, pigmentary changes, proteinuria, pruritus, pseudomembranous colitis, renal failure, respiratory failure, respiratory fibrosis, skin necrosis, skin ulceration, speech impairment (aphasia, dysarthria), Stevens-Johnson syndrome, stomatitis, suppressed hematopoiesis (blood cell formation), telangiectasia, thrombocytopenia, thromboembolic events (e.g., arterial thrombosis, cerebral thrombosis, deep vein thrombosis, pulmonary embolus, retinal vein thrombosis, thrombophlebitis), toxic epidermal necrolysis, transient blindness, tumor lysis syndrome, urticaria, vaginal discharge, vomiting.
Antidote
Discontinue methotrexate and notify the physician of any side effects. Leucovorin calcium (citrovorum factor, folinic acid) may be given PO, IM, or IV promptly to counteract inadvertent overdose. Leucovorin calcium is also indicated as a planned rescue mechanism for large doses of methotrexate required to treat some malignancies. Doses equal to dose of methotrexate are frequently required. Should be given within 1 hour in overdose, 24 hours in rescue. See specific process for overdose and for rescue for high-dose MTX in leucovorin calcium monograph. Doses up to 150 mg or 100 mg/M2 every 3 hours may be required if SCr is 50% or greater than baseline measurement before methotrexate administration. Serum methotrexate must come down to below 0.05 micromolar. Continuing hydration and urinary alkalinization are mandatory to prevent precipitation in renal tubules. Monitor fluid and electrolyte status until serum methotrexate has fallen to less than 0.05 micromolar and renal failure has resolved. Glucarpidase (Voraxaze), an antidote indicated for the treatment of toxic methotrexate concentration in patients with delayed methotrexate clearance due to impaired renal function, may also be used. If glucarpidase is used, do not administer leucovorin within 2 hours before or after a dose of glucarpidase because leucovorin is a substrate for glucarpidase; see glucarpidase monograph. Administration of whole blood products (e.g., packed RBCs, platelets, leukocytes) and/or blood modifiers (e.g., darbepoetin alfa [Aranesp], epoetin alfa [Epogen], filgrastim [Neupogen, Zarxio], pegfilgrastim [Neulasta], sargramostim [Leukine]) may be indicated to treat bone marrow toxicity. Death may occur from the progression of most of these side effects. Symptomatic and supportive therapy is indicated. Charcoal hemoperfusion may be helpful, and/or acute intermittent hemodialysis with a high-flux dialyzer may be used to counteract toxicity or inadvertent overdose.
Methylergonovine maleate
(meth-ill-er-GON-oh-veen MAL-ee-ayt)
Methergine
Uterine stimulant (oxytocic)
pH 2.7 to 3.5
Usual dose
1 mL (0.2 mg); repeat doses should be IM or PO.
Dilution
Check expiration date on vial; methylergonovine deteriorates with age. May be given undiluted. Some clinicians recommend dilution with 5 mL of NS. Do not add to IV solutions. May be given through Y-tube or three-way stopcock of infusion set.
Filters:
No data available from manufacturer; suggests following hospital protocol for filtering from ampules.
Storage:
Store in refrigerator (2° to 8° C [36° to 46° F]); protect from light.
Compatibility
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
One source suggests the following compatibilities:
Y-site:
Heparin, hydrocortisone sodium succinate (Solu-Cortef).
Rate of administration
0.2 mg or fraction thereof over 1 minute. Too-rapid injection may cause severe nausea and vomiting. See Precautions.
Actions
A semi-synthetic ergot alkaloid used for prevention and control of postpartum hemorrhage. An oxytocic. It exerts a direct stimulation on the smooth muscle of the uterus. Increases tone, rate, and amplitude of rhythmic contractions. Shortens third-stage labor and reduces blood loss. In therapeutic doses the prolonged initial contraction of the uterus is followed by periods of relaxation and contraction. May also produce vasoconstriction, increase CVP and BP, and may rarely produce peripheral ischemia. Effective within minutes. Half-life approximately 3.4 hours. It is probably metabolized in the liver and excreted in feces. Secreted in breast milk.
Indications and uses
Routine management of uterine atony, hemorrhage, and subinvolution of the uterus following delivery of the placenta. ■ Control of uterine hemorrhage in the second stage of labor following delivery of the anterior shoulder.
Contraindications
Hypersensitivity, hypertension, pregnancy before third stage of labor (delivery of the placenta) except as stated in Indications, toxemia. ■ Contraindicated for concomitant use with potent CYP3A4 inhibitors (e.g., protease inhibitors [ritonavir (Norvir)], macrolide antibiotics [erythromycin], and azole antifungals [ketoconazole (Nizoral)]); see Drug/Lab Interactions.
Precautions
IV administration is for emergency use only. IM or oral routes are preferred and should be used after the initial IV dose. ■ IV use may induce hypertension and/or CVA. Give slowly and monitor BP. ■ Use caution in presence of sepsis, obliterative vascular disease, and hepatic or renal disease. ■ Patients with coronary artery disease or risk factors for coronary artery disease (e.g., diabetes, high cholesterol, obesity, smoking) may be more susceptible to developing myocardial ischemia and infarction associated with methylergonovine-induced vasospasm. ■ Use with caution during the second stage of labor. The necessity for manual removal of a retained placenta should occur rarely with proper technique and adequate allowance of time for a spontaneous separation. ■ Avoid intra-arterial or peri-arterial injection. ■ See Contraindications.
Monitor:
Monitor BP. ■ Observe for signs of excessive bleeding. ■ See Drug/Lab Interactions.
Maternal/child:
Category C: see Contraindications and Precautions. ■ Avoid breast-feeding during treatment with methylergonovine. Wait at least 12 hours after administration of the last dose before initiating or resuming breast-feeding. Milk secreted during this period should be discarded. May be given orally with caution during breast-feeding for up to 1 week after delivery. ■ Inadvertent administration of methylergonovine to newborn infants resulting in convulsions, cyanosis, oliguria, and respiratory depression has been reported. Methylergonovine should be stored separately from medications intended for neonatal administration (e.g., vitamin K, hepatitis B vaccine). ■ Safety and effectiveness for use in pediatric patients not established.
Elderly:
Difference in safety and effectiveness compared to younger adults not observed. ■ If used in the elderly, dose selection should be cautious.
Drug/lab interactions
Severe hypertension and cerebrovascular accidents can result with ephedrine and other vasopressors. Hydralazine IV will reduce this hypertension. ■ Do not administer with potent CYP3A4 inhibitors, such as macrolide antibiotics (e.g., erythromycin [Erythrocin], troleandomycin [TAO], clarithromycin [Biaxin]), HIV protease inhibitors (e.g., indinavir [Crixivan], nelfinavir [Viracept], ritonavir [Norvir]), reverse transcriptase inhibitors (e.g., delavirdine [Rescriptor]), or azole antifungals (e.g., ketoconazole [Nizoral], voriconazole [VFEND]). Serum levels increased. Elevated levels of ergot alkaloids can cause ergotism (i.e., risk for vasospasm potentially leading to cerebral ischemia and/or ischemia of the extremities). ■ May be used cautiously with less potent CYP3A4 inhibitors (e.g., saquinavir [Invirase], nefazodone, fluconazole [Diflucan], grapefruit juice, fluoxetine [Prozac], fluvoxamine [Luvox], zileuton [Zyflo], and clotrimazole [Gyne-Lotrimin, Mycelex]). ■ Strong CYP3A4 inducers (e.g., rifampin [Rifadin], nevirapine [Viramune]) may decrease effectiveness of methylergonovine. ■ Anesthetics (e.g., halothane, methoxyflurane) may reduce oxytocic effect of methylergonovine. ■ Produces vasoconstriction and can be expected to reduce the effect of antianginal drugs (e.g., nitroglycerin). ■ Concomitant use with other ergot alkaloids or prostaglandins will produce additive effects. Use caution.
Side effects
Rare in therapeutic doses but may include the following:
Abdominal pain (caused by uterine contractions), bradycardia, chest pain (temporary), coronary artery spasm, diaphoresis, diarrhea, dilated pupils, dizziness, dyspnea, hallucinations, headache, hematuria, hypersensitivity reactions, hypertension (transient), hypotension, leg cramps, MI, nasal congestion, nausea, palpitations, rash, seizures, tachycardia, thrombophlebitis, tinnitus, vasoconstriction, vasospasm, vomiting, water intoxication, weakness.
Overdose:
Cerebrovascular accident, coma, convulsions, hypertension (followed by hypotension in severe cases), hypothermia, numbness, oliguria, palpitations, respiratory depression, severe nausea and vomiting, tachycardia, tingling of the extremities.
Post-marketing:
Angina, atrioventricular block, cerebrovascular accident, paresthesia, ventricular fibrillation, ventricular tachycardia.
Antidote
Discontinue the drug immediately at the onset of any side effect and notify the physician. Most side effects are transient unless there is severe toxicity and will be treated symptomatically. Use antiemetics (e.g., prochlorperazine [Compazine], ondansetron [Zofran]) for nausea and vomiting. Treat seizures with anticonvulsants (e.g., diazepam [Valium], phenytoin [Dilantin]). Maintain adequate pulmonary ventilation, especially if convulsions or coma develop. Correct hypotension with pressor drugs (e.g., dopamine). Apply warmth to extremities to control peripheral vasospasm.
Methylprednisolone sodium succinate
(meth-ill-pred-NISS-oh-lohn SO-dee-um SUK-sih-nayt)
A-Methapred, Solu-Medrol
Hormone (corticosteroid)
Anti-inflammatory
pH 7 to 8
Usual dose
Use the lowest possible dose to control the condition being treated. When reduction in dose is possible, the reduction should be gradual.
Average dose range is 10 to 40 mg initially. May be repeated every 4 to 6 hours as necessary. IV methylprednisolone is usually given in an emergency situation or when oral dosing is not feasible. Larger doses may be justified by patient condition. Repeat until adequate response, then decrease dose as indicated. Total dose usually does not exceed 1.5 Gm/24 hr, but higher doses have been used in life-threatening shock. High-dose treatment is used until patient condition stabilizes, usually no longer than 48 to 72 hours.
Anti-inflammatory:
10 to 40 mg. May be repeated every 4 to 6 hours as necessary.
Acute spinal cord injury high-dose therapy (unlabeled):
Spinal cord injury must be less than 8 hours old and above L-2. The earlier methylprednisolone therapy begins, the better the results.
Loading dose:
30 mg/kg of a specifically diluted solution (see Dilution) evenly distributed over 15 minutes. Maintain IV line with standard IV fluids for 45 minutes, then begin a maintenance dose of 5.4 mg/kg/hr for 23 hours. Discontinue 24 hours after loading dose initiated.
Status asthmaticus:
Newer asthma guidelines recommend 40 to 80 mg/day in 1 to 2 divided doses until peak expiratory flow is 70% of predicted or personal best. Another source recommends a loading dose of 2 mg/kg followed by 0.5 to 1 mg/kg/dose every 6 hours for up to 5 days.
Acute exacerbation of multiple sclerosis:
160 mg as a single dose each day for 7 days. Follow with 64 mg every other day for 1 month.
Pneumocystis jiroveci pneumonia (unlabeled):
Initiate within 24 to 72 hours of initial antibiotic PCP therapy. 30 mg twice daily for 5 days. Follow with 30 mg once daily for 5 days (Days 6 to 10). Then reduce to 15 mg once daily for 11 days (Days 11 to 21) or until antibiotic regimen is complete.
Severe lupus nephritis (unlabeled):
1 Gm as an infusion over 1 hour for 3 days. Follow with long-term prednisolone oral therapy.
Pediatric dose
See Maternal/Child. Dose may be reduced for infants and pediatric patients but should be governed more by the severity of the condition and the response of the patient than by age or size. Dose should not be less than 0.5 mg/kg every 24 hours.
Anti-inflammatory/immunosuppressive:
The range of initial doses is 0.11 to 1.6 mg/kg/day (3.2 to 48 mg/M2/day) in 3 to 4 divided doses (0.0275 to 0.4 mg/kg every 6 hours or 0.036 to 0.53 mg/kg every 8 hours, or 0.8 to 12 mg/M2 every 6 hours or 1.06 to 16 mg/M2 every 8 hours). Another source recommends 0.5 to 1.7 mg/kg/day or 5 to 25 mg/M2/day in divided doses every 6 to 12 hours (0.125 to 0.425 mg/kg or 1.25 to 6.25 mg/M2 every 6 hours, or 0.25 to 0.85 mg/kg or 2.5 to 12.5 mg/M2 every 12 hours).
Status asthmaticus:
Newer asthma guidelines recommend 0.5 to 1 mg/kg every 12 hours (maximum 60 mg/day) until peak expiratory flow is 80% of predicted or personal best. Another source recommends 2 mg/kg as a loading dose. Maintain with 0.5 to 1 mg/kg every 6 hours for up to 5 days.
Severe lupus nephritis (unlabeled):
30 mg/kg every other day for 6 doses. Follow with long-term prednisolone oral therapy.
Dose adjustments
Reduced dose may be required; see Precautions and Drug/Lab Interactions. ■ Clearance of corticosteroids is decreased in patients with hypothyroidism and increased in patients with hyperthyroidism. Dose adjustment may be required.
Dilution
Available in Act-O-Vials containing 40 mg, 125 mg, 500 mg, and 1,000 mg. Each vial has an appropriate amount of diluent. Reconstitute by pressing down on the plastic activator, allowing the diluent into the lower chamber. Agitate gently. Remove the plastic tab covering the center of the stopper and sterilize the stopper with a suitable germicide. Using sterile technique, insert a needle through the center of the rubber stopper to withdraw diluted solution. To be diluted only with diluent supplied in Act-O-Vial. Also available in vials, including a 2,000-mg dose. Should be diluted with accompanying diluent or BWFI with benzyl alcohol. May be given as direct IV, as an infusion, or further diluted in desired amounts of D5W, D5NS, or NS.
Acute spinal cord injury loading and maintenance doses:
Each 1-Gm vial must be diluted to 16 mL with bacteriostatic water to maintain potency and avoid precipitation (62.5 mg/mL). Further dilute in D5W, D5NS, or NS with an amount to facilitate dose of 5.4 mg/kg/hr. (Example for a patient weighing 50 kg: [50 kg × 5.4 mg/hr = 270 mg/hr. 270 mg/hr × 23 hours = 6,210 mg total dose]. With a total dose of 6,210 mg at 62.5 mg/mL, you will have 99.36 [100] mL of reconstituted methylprednisolone. Add an additional 100 mL diluent to achieve 31.25 mg/mL. 270 mg/hr is the desired dose for this patient. 270 mg/hr divided by 31.25 mg/mL [strength of solution] equals 8.6. Administer at 8.6 mL/hr to achieve desired dose over 23 hours.)
Storage:
Protect from light. Store both unreconstituted product and solution at RT (20° to 25° C [68° to 77° F]). Use solution within 48 hours of mixing. Heat sensitive; do not autoclave.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer states, “Because of possible physical incompatibilities, should not be diluted or mixed with other solutions.”
One source suggests the following compatibilities:
Additive:
Aminophylline, chloramphenicol (Chloromycetin), clindamycin (Cleocin), cytarabine (ARA-C), dopamine, granisetron (Kytril), heparin, norepinephrine (Levophed), penicillin G potassium, ranitidine (Zantac), theophylline, verapamil.
Y-site:
Acetaminophen (Ofirmev), acyclovir (Zovirax), alprostadil, amifostine (Ethyol), amiodarone (Nexterone), amphotericin B cholesteryl (Amphotec), anidulafungin (Eraxis), aztreonam (Azactam), bivalirudin (Angiomax), cefepime (Maxipime), ceftaroline (Teflaro), ceftazidime (Fortaz), cisatracurium (Nimbex), cladribine (Leustatin), cytarabine (ARA-C), dexmedetomidine (Precedex), diltiazem (Cardizem), dopamine, doripenem (Doribax), doxorubicin liposomal (Doxil), enalaprilat (Vasotec IV), famotidine (Pepcid IV), fludarabine (Fludara), granisetron (Kytril), heparin, hetastarch in electrolytes (Hextend), 6% hydroxyethyl starch (Voluven), linezolid (Zyvox), melphalan (Alkeran), meperidine (Demerol), methotrexate, metronidazole (Flagyl IV), midazolam (Versed), milrinone (Primacor), morphine, nicardipine (Cardene IV), oxaliplatin (Eloxatin), pemetrexed (Alimta), piperacillin/tazobactam (Zosyn), potassium chloride (KCl), remifentanil (Ultiva), sodium bicarbonate, tacrolimus (Prograf), telavancin (Vibativ), teniposide (Vumon), theophylline, thiotepa, topotecan (Hycamtin).
Rate of administration
IV injection:
A single dose of 10 to 40 mg over several minutes. When higher doses are needed, administer up to 30 mg/kg over 30 minutes or more. Too-rapid administration of high doses (greater than 500 mg administered over a period of less than 10 minutes) may precipitate hypotension, cardiac arrhythmia, and sudden death. Direct IV administration of lower doses (10 to 40 mg) is usually the route of choice and eliminates the possibility of overloading the patient with IV fluids. May be given as an infusion in its own diluent. At the discretion of the physician, a continuous infusion may be given, properly diluted, over a specified time. Another source suggests the following:
A single dose of up to 1.8 mg/kg or 125 mg may be given IV push over 3 to 15 minutes.
A single dose of 2 mg/kg or 250 mg or more may be given as an infusion over 15 to 30 minutes.
A single dose of 15 mg/kg or greater than 500 mg may be given as an infusion over 30 minutes or more.
Acute spinal cord injury:
See Usual Dose and Dilution.
Actions
A glucocorticoid steroid with potent metabolic, anti-inflammatory actions and innumerable other effects. Has a greater anti-inflammatory potency than prednisolone and less tendency to cause excessive potassium and calcium excretion and sodium and water retention. Has five times the potency of hydrocortisone sodium succinate. Has minimal mineralocorticoid activity. Primarily used for anti-inflammatory and immunosuppressive effects. Demonstrable effects seen within 1 hour of administration. Primarily metabolized in the liver and excreted in the urine and feces. Dose almost completely excreted after 12 hours, which allows the use of very large doses with reasonable safety. Crosses the placental barrier. Secreted in breast milk.
Indications and uses
Includes treatment of allergic states, dermatologic diseases, endocrine disorders, gastrointestinal diseases, hematologic disorders, neoplastic diseases, nervous system disorders, ophthalmic diseases, renal diseases, respiratory diseases, and rheumatic disorders. See prescribing information for a complete list. Used primarily as an anti-inflammatory or immunosuppressant agent. May be used in conjunction with other forms of therapy, such as epinephrine for acute hypersensitivity reactions. Oral therapy should be used when appropriate.
Unlabeled uses:
High-dose therapy as an adjunct to traditional spinal cord injury management; to improve neurologic recovery in an acute (less than 8 hours old) spinal cord injury above L-2. ■ Treatment of Pneumocystis jiroveci pneumonia as an adjunct to antibiotics.
Contraindications
Absolute contraindications in long-term therapy, except in life-threatening situations:
Hypersensitivity to any product component, including sulfites; systemic fungal infections. ■ Formulations containing benzyl alcohol are contraindicated in neonates and for intrathecal administration.
Relative contraindications:
Active or healed tuberculosis, amebiasis (latent or active), cerebral malaria, chickenpox, ocular herpes simplex, pregnancy.
Precautions
Not the drug of choice to treat acute adrenocortical insufficiency. ■ May produce hypothalamic-pituitary-adrenal (HPA) axis suppression with resulting glucocorticosteroid insufficiency in patients undergoing chronic or prolonged therapy. To avoid relative adrenocortical insufficiency, do not stop therapy abruptly; taper off. Patient is observed carefully, especially under stress, for up to 2 years; exception is very short-term therapy. ■ Increased doses of rapidly acting corticosteroids are indicated when patients on corticosteroid therapy are subjected to any unusual stress (e.g., surgery, hospitalization).These increased doses should be used before, during, and after the stressful situation. ■ Formulation may contain benzyl alcohol; see Contraindications and Maternal/Child. ■ Rare instances of anaphylactoid reactions have been reported in patients receiving corticosteroid therapy. ■ In one study, an increase in mortality was seen in patients with cranial trauma who had no other clear indication for corticosteroid treatment. Should not be used for treatment of traumatic brain injury. ■ Use with caution in patients who have had a recent MI. Ventricular free wall rupture has been reported. ■ Patients taking corticosteroids may be more susceptible to infections. Latent disease may be activated. Intercurrent infections may be exacerbated; see Contraindications. ■ Use with caution in patients with CHF, hypertension, or renal insufficiency. May affect fluid and electrolyte balance. ■ Use with caution in patients with thyroid dysfunction; see Dose Adjustments. ■ Use with caution in patients with active or latent peptic ulcers, diverticulitis, fresh intestinal anastomoses, and nonspecific ulcerative colitis. May be at increased risk for perforation. ■ Metabolism of corticosteroids is decreased in patients with cirrhosis; effects may be enhanced. ■ Use with caution in patients at risk for osteoporosis. Corticosteroids decrease bone formation, increase bone resorption, and decrease protein matrix of the bone. May lead to inhibition of bone growth in pediatric patients and the development of osteoporosis at any age. ■ An acute myopathy has been reported with the use of high doses of corticosteroids. Most often seen in patients with disorders of neuromuscular transmission (e.g., myasthenia gravis) or in patients receiving concomitant therapy with neuromuscular blocking drugs. Myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Clinical improvement following discontinuation of corticosteroids may take weeks to years. ■ May induce psychological changes (e.g., depression, euphoria, insomnia, mood swings, personality changes, psychosis). May aggravate existing emotional instability or psychotic tendencies. ■ Kaposi’s sarcoma has been reported in patients receiving corticosteroid therapy, most often for chronic conditions. Clinical improvement has been seen with discontinuation of the corticosteroid. ■ Prophylactic antacids may prevent peptic ulcer complications.
Monitor:
Monitor electrolytes. May cause sodium retention and potassium and calcium excretion. ■ May cause hypertension secondary to fluid and electrolyte disturbances. Monitor BP. ■ May mask signs of infection. ■ May increase insulin needs in patients with diabetes. Monitor serum glucose. ■ Administer single dose before 9 am to reduce suppression of adrenocortical activity. ■ During prolonged therapy, routine laboratory studies such as urinalysis, 2-hour postprandial blood sugar, BP, body weight assessment, and chest x-ray should be obtained at regular intervals. Upper GI x-rays are suggested for patients with a history of ulcers or significant dyspepsia. ■ May increase intraocular pressure. Periodic ophthalmic exams may be necessary with prolonged treatment. ■ See Drug/Lab Interactions.
Patient education:
Report edema, tarry stools, or weight gain promptly. Report anorexia, diarrhea, dizziness, fatigue, low blood sugar, nausea, weakness, weight loss, or vomiting; may indicate adrenal insufficiency after dose reduction or discontinuing therapy. ■ May mask signs of infection and/or decrease resistance. ■ Diabetics may have increased requirement for insulin or oral hypoglycemics. ■ Avoid immunizations with live virus vaccines. ■ Avoid exposure to measles or chickenpox. Seek immediate medical advice if exposure occurs. ■ Carry ID stating steroid dependent if receiving prolonged therapy.
Maternal/child:
Category C: corticosteroids have been shown to be teratogenic in many species. ■ Discontinue breast-feeding. ■ Infants born to mothers who received corticosteroids during pregnancy should be carefully monitored for signs of hypoadrenalism. ■ May contain benzyl alcohol, which has been associated with a fatal “gasping syndrome” in neonates. ■ Monitor growth and development of pediatric patients receiving prolonged treatment.
Elderly:
Differences in response between the elderly and younger patients have not been identified. Dose selection should be cautious based on the possibility of age-related organ impairment (e.g., bone marrow reserve, renal, hepatic). May be more sensitive to effects.
Drug/lab interactions
Aminoglutethimide (Cytadren) may lead to a loss of corticosteroid-induced adrenal suppression. ■ Metabolism increased and effects reduced by hepatic enzyme–inducing agents (e.g., alcohol, barbiturates [e.g., phenobarbital], hydantoins [e.g., phenytoin (Dilantin)], rifampin [Rifadin]); dose adjustments may be required when adding or deleting from drug profile. ■ Risk of hypokalemia increased with amphotericin B or potassium-depleting diuretics (e.g., thiazides, furosemide, ethacrynic acid). Monitor potassium levels and cardiac function. Increased risk of digoxin toxicity secondary to hypokalemia. ■ May decrease effectiveness of potassium supplements; monitor serum potassium. ■ Diuretics decrease sodium and fluid retention effects of corticosteroids; corticosteroids decrease sodium excretion and diuretic effects of diuretics. ■ Use with cyclosporine in organ transplants is therapeutic but may increase cyclosporine toxicity; seizures have been reported; use caution. ■ Clearance decreased and effects increased with estrogens, oral contraceptives, triazole antifungals (e.g., itraconazole [Sporanox]), and macrolide antibiotics (e.g., azithromycin [Zithromax], erythromycin, troleandomycin [TAO]). ■ Coadministration of aprepitant (fosaprepitant [Emend]) may increase methylprednisolone levels. Dose reduction of methylprednisolone may be indicated. ■ May interact with anticoagulants, nondepolarizing muscle relaxants (e.g., atracurium [Tracrium]), or theophyllines; may inhibit or potentiate action; monitor carefully. ■ Monitor patients receiving antidiabetic agents (e.g., insulin, glyburide) or thyroid hormones carefully; dose adjustments of either or both agents may be required. ■ May antagonize effects of isoniazid and salicylates; dose adjustments may be required. ■ Administration of live virus or live-attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. Inactivated vaccines may be administered, but the response to such vaccines cannot be predicted. ■ Concomitant use with NSAIDs (e.g., ibuprofen [Advil, Motrin], naproxen [Aleve, Naprosyn]) may increase the risk of adverse GI effects. ■ Concomitant use with anticholinesterase agents (e.g., neostigmine) may produce severe weakness in patients with myasthenia gravis. If possible, anticholinesterase agents should be withdrawn at least 24 hours before initiating corticosteroid therapy. ■ Altered protein-binding capacity will impact effectiveness of this drug. ■ Corticosteroids may suppress reactions to skin tests. ■ See Dose Adjustments.
Side effects
Do occur but are usually reversible: Cushing’s syndrome; electrolyte and calcium imbalance; euphoria; glycosuria; hyperglycemia; hypersensitivity reactions, including anaphylaxis; hypertension; increased appetite; increased intracranial pressure; menstrual irregularities; peptic ulcer perforation and hemorrhage; protein catabolism; spontaneous fractures; transitory burning or tingling, sweating, headache, or weakness; thromboembolism; and many others.
Antidote
Notify the physician of any side effect. Will probably treat the side effect if necessary. Resuscitate as necessary for anaphylaxis and notify physician. Keep epinephrine immediately available.
Metoclopramide hydrochloride 
(meh-toe-kloh-PRAH-myd hy-droh-KLOR-eyed)
Reglan
GI stimulant
Antiemetic
pH 4.5 to 6.5
Usual dose
Small bowel intubation and/or radiologic examination of the small bowel:
10 mg (2 mL) as a single dose.
Antiemetic:
High-dose regimen for highly emetogenic chemotherapy is rarely used; 5HT3 receptor antagonists (e.g., granisetron [Kytril], ondansetron [Zofran]) preferred. 2 mg/kg of body weight 30 minutes before giving emetogenic cancer chemotherapy (e.g., cisplatin, dacarbazine). Repeat every 2 hours for 2 doses, then every 3 hours for 3 doses; see Dose Adjustments. For less emetogenic regimens, 1 mg/kg/dose may be adequate.
Diabetic gastroparesis:
10 mg immediately before each meal and at bedtime. Use IV for up to 10 days if symptoms are severe. Continue treatment PO for 2 to 8 weeks.
Prevention of postoperative nausea and vomiting:
10 mg, usually given IM toward the end of surgery. Up to 20 mg may be used.
Pediatric dose
Small bowel intubation and/or radiologic examination of the small bowel:
6 to 14 years:
2.5 to 5 mg.
Under 6 years:
0.1 mg/kg of body weight.
Gastroesophageal reflux or GI dysmotility (unlabeled):
0.1 to 0.2 mg/kg/dose. May be given every 6 hours if required. Maximum dose 0.8 mg/kg/24 hr (0.2 mg/kg/dose every 6 hours).
Antiemetic (unlabeled):
Rarely used. 5HT3-receptor antagonists preferred. A high-dose regimen for highly emetogenic chemotherapy of 1 to 2 mg/kg/dose every 2 to 6 hours has been administered but is rarely used. Premedicate with diphenhydramine (Benadryl) to reduce extrapyramidal symptoms.
Dose adjustments
Antiemetic dose may be reduced to 1 mg/kg if initial doses suppress vomiting. Initial doses may be reduced to 1 mg/kg for less emetogenic regimens. ■ Reduce initial dose by half in any patient with a CrCl less than 40 mL/min. Adjust subsequent doses as indicated. ■ Caution and lower-end dosing suggested in elderly patients. Consider potential for decreased organ function and concomitant disease or drug therapy. ■ See Drug/Lab Interactions.
Dilution
May be given undiluted if dose does not exceed 10 mg. For doses exceeding 10 mg dilute in at least 50 mL of D5W, NS, D5/1/2NS, R, or LR, and give as an infusion.
Storage:
Light sensitive; store in carton before use. Diluted solutions stable for 24 hours in normal light, 48 hours if protected from light. Do not freeze unless diluted in NS. Discard if color or particulate matter is observed.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer lists as incompatible with chloramphenicol (Chloromycetin) and sodium bicarbonate.
Sources suggest the following compatibilities:
Additive:
Manufacturer lists ampicillin, ascorbic acid, benztropine (Cogentin), cisplatin, clindamycin (Cleocin), cyclophosphamide (Cytoxan), cytarabine (ARA-C), dexamethasone (Decadron), diphenhydramine (Benadryl), doxorubicin (Adriamycin), erythromycin (Erythrocin), heparin, insulin (regular), lidocaine, mannitol, methotrexate, multivitamins (M.V.I.), penicillin G potassium, potassium acetate, and potassium phosphates. Other sources add meperidine (Demerol), meropenem (Merrem IV), morphine, potassium chloride (KCl), verapamil.
Y-site:
All drugs listed by the manufacturer as compatible under Additive. Other sources add acetaminophen (Ofirmev), acyclovir (Zovirax), aldesleukin (Proleukin), amifostine (Ethyol), aztreonam (Azactam), bivalirudin (Angiomax), bleomycin (Blenoxane), ceftaroline (Teflaro), ciprofloxacin (Cipro IV), cisatracurium (Nimbex), cisplatin, cladribine (Leustatin), cyclophosphamide (Cytoxan), dexmedetomidine (Precedex), diltiazem (Cardizem), docetaxel (Taxotere), doripenem (Doribax), doxapram (Dopram), doxorubicin (Adriamycin), droperidol (Inapsine), etoposide phosphate (Etopophos), famotidine (Pepcid IV), fenoldopam (Corlopam), fentanyl, filgrastim (Neupogen), fluconazole (Diflucan), fludarabine (Fludara), fluorouracil (5-FU), foscarnet (Foscavir), gallium nitrate (Ganite), gemcitabine (Gemzar), granisetron (Kytril), heparin, hetastarch in electrolytes (Hextend), hydromorphone (Dilaudid), idarubicin (Idamycin), leucovorin calcium, levofloxacin (Levaquin), linezolid (Zyvox), melphalan (Alkeran), meperidine (Demerol), meropenem (Merrem IV), methadone (Dolophine), methotrexate, mitomycin (Mutamycin), morphine, ondansetron (Zofran), oxaliplatin (Eloxatin), paclitaxel (Taxol), palonosetron (Aloxi), pemetrexed (Alimta), piperacillin/tazobactam (Zosyn), quinupristin/dalfopristin (Synercid), remifentanil (Ultiva), sargramostim (Leukine), tacrolimus (Prograf), telavancin (Vibativ), teniposide (Vumon), thiotepa, tigecycline (Tygacil), topotecan (Hycamtin), vinblastine, vincristine, vinorelbine (Navelbine), zidovudine (AZT, Retrovir).
Rate of administration
Too-rapid IV injection will cause intense anxiety, restlessness, and then drowsiness.
IV injection:
10 mg or fraction thereof over 2 minutes. Reduce rate of injection in pediatric patients.
Infusion:
Administer over a minimum of 15 minutes.
Actions
A dopamine antagonist. Antiemetic properties appear to be the result of antagonism of central and peripheral dopamine receptors. Blocks the stimulation of medullary chemoreceptor trigger zones by dopamine. Inhibits nausea and vomiting. Increases tone and amplitude of gastric contractions, relaxes the lower pyloric sphincter and duodenal bulb, and increases peristalsis of the duodenum and jejunum, resulting in accelerated gastric emptying. Does not stimulate gastric, biliary, or pancreatic secretions. Acts even if vagal innervation not present. Action negated by anticholinergic drugs. Distributes extensively into tissues. Onset of action occurs in 1 to 3 minutes and lasts 1 to 2 hours. Average half-life is 5 to 6 hours. Excreted in urine. Secreted in breast milk.
Indications and uses
Facilitate small bowel intubation. ■ Stimulate gastric and intestinal emptying of barium to permit radiologic examination of the stomach and small intestine. ■ Prevention of nausea and vomiting associated with emetogenic cancer chemotherapy. ■ Prophylaxis of postoperative nausea and vomiting when nasogastric suction is not indicated. ■ Diabetic gastroparesis.
Contraindications
Situations in which gastric motility is contraindicated (i.e., gastric hemorrhage, obstruction, or perforation); known hypersensitivity to metoclopramide; patients with epilepsy or patients taking drugs that may also cause extrapyramidal reactions; pheochromocytoma.
Precautions
May produce sedation, extrapyramidal symptoms, or Parkinson-like symptoms, similar to those seen with phenothiazines. Use caution in patients with pre-existing disease. ■ Acute dystonic reactions (a type of extrapyramidal symptom [EPS]) are usually seen during the first 24 to 48 hours of treatment and are more common in pediatric patients, in adults under 30 years of age, and at higher doses used for prophylaxis of N/V due to chemotherapy. ■ Tardive dyskinesia may develop and is usually related to duration of treatment and total cumulative dose. Avoid use for longer than 12 weeks unless benefit outweighs risk of tardive dyskinesia. ■ Neuroleptic malignant syndrome (NMS) has been reported rarely. Potentially fatal. Discontinue metoclopramide immediately; see Monitor. ■ Produces a transient increase in plasma aldosterone; patients with cirrhosis or CHF may develop fluid retention and volume overload. If S/S occur, discontinue metoclopramide. ■ Use with caution in patients with hypertension. May cause release of catecholamines, exacerbating the condition. ■ A prolactin-elevating compound; may be carcinogenic. Risk with a single dose almost nonexistent. ■ May cause serious depression and suicidal tendencies; use extreme caution in any patient with a history of depression. ■ Patients with NADH-cytochrome b5 reductase deficiency are at increased risk for developing methemoglobinemia and/or sulfhemoglobinemia. In patients with G6PD deficiency who develop methemoglobinemia, methylene blue treatment is not recommended. Can cause hemolytic anemia. ■ See Maternal/Child.
Monitor:
Monitor vital signs. ■ Pretreatment with diphenhydramine may reduce incidence of extrapyramidal symptoms with larger doses (e.g., antiemetic). ■ Monitor for S/S of NMS (e.g., hyperthermia, muscle rigidity, altered consciousness, and evidence of autonomic instability [irregular pulse or BP, tachycardia, diaphoresis, and arrhythmias]). ■ Discontinue therapy in patients who develop S/S of tardive dyskinesia (syndrome of potentially irreversible involuntary movements of the tongue, face, mouth, jaw, trunk, or extremities). ■ See Precautions and Drug/Lab Interactions.
Patient education:
Use caution performing any task that requires alertness, coordination, or physical dexterity; may produce dizziness and drowsiness. ■ If any involuntary movement of eyes, face, or limbs occurs, notify physician promptly. ■ Avoid alcohol or other CNS depressants (e.g., barbiturates, benzodiazepines [e.g., diazepam (Valium)]).
Maternal/child:
Category B: use caution in pregnancy and breast-feeding. ■ May increase milk production (elevates prolactin). ■ Pharmacokinetics highly variable in children and neonates. ■ Safety and effectiveness for use in pediatric patients not established except when administered to facilitate small bowel intubation. ■ Dystonic reactions are more common in pediatric patients. ■ Prolonged clearance in neonates may produce excessive serum concentrations. ■ May cause methemoglobinemia in premature and full-term neonates at doses exceeding 0.5 mg/kg/24 hr. ■ See Precautions and Side Effects.
Elderly:
May be more sensitive to therapeutic or adverse effects. ■ Long-term use increases risk of extrapyramidal effects (e.g., parkinsonism, tardive dyskinesia). ■ See Dose Adjustments and Precautions.
Drug/lab interactions
Antagonized by anticholinergic drugs (e.g., atropine) and narcotic analgesics (e.g., morphine). ■ May potentiate alcohol and cyclosporine. ■ Drugs ingested orally may be absorbed more slowly or more rapidly depending on the absorption site (e.g., inhibits cimetidine, digoxin). ■ Potentiates MAO inhibitors (e.g., selegiline [Eldepryl]); use extreme caution or do not use. ■ Insulin reactions may result from gastric stasis, making diabetic control difficult. Dose or timing of insulin may need adjustment. ■ Extrapyramidal effects may be potentiated with concomitant use of phenothiazines, butyrophenones, and thioxanthines (antipsychotic drugs). ■ Used concurrently, metoclopramide and levodopa have opposite effects on dopamine receptors; metoclopramide is inhibited and levodopa is potentiated.
Side effects
Usually mild, transient, and reversible after metoclopramide is discontinued. Hypersensitivity reactions can occur. Acute CHF, anxiety, arrhythmias, bowel disturbances, confusion, convulsions, depression (severe, may have suicidal tendencies), dizziness, drowsiness, extrapyramidal reactions, fatigue, fluid retention, hallucinations, headache, hypertension, hypotension, insomnia, methemoglobinemia in neonates, nausea, NMS (hyperthermia, muscle rigidity, altered consciousness, and evidence of autonomic instability [irregular pulse or BP, tachycardia, diaphoresis, and arrhythmias]), restlessness, sulfhemoglobinemia in adults, tardive dyskinesia. Numerous other side effects may occur.
Overdose:
Disorientation, drowsiness, and extrapyramidal reactions.
Antidote
Notify physician of all side effects. Most will respond to a reduced dose or discontinuation of metoclopramide. Treat overdose or extrapyramidal reactions with diphenhydramine (Benadryl) or benztropine (Cogentin). Symptoms should disappear within 24 hours. To manage NMS, immediately discontinue metoclopramide. Intensive symptomatic treatment and monitoring are required. Bromocriptine (Parlodel) and dantrolene (Dantrium) have been used to treat NMS, but effectiveness not established. Discontinue therapy in patients who develop S/S of tardive dyskinesia; symptoms may resolve. Treat methemoglobinemia with IV methylene blue. Hemodialysis is not likely to be useful in an overdose. Resuscitate as necessary.
Metoprolol tartrate 
(me-toe-PROH-lohl TAHR-trayt)
Lopressor
Beta-adrenergic blocking agent
Antiarrhythmic (post MI)
pH 7.5
Usual dose
Treatment of myocardial infarction:
5 mg as an IV bolus dose. Initiate as soon as the patient’s hemodynamic condition has stabilized. Repeat at 2-minute intervals for 2 more doses; a total dose of 15 mg (AHA recommends 5 mg at 5-minute intervals to a total dose of 15 mg). If IV doses are well tolerated, give 50 mg PO every 6 hours for 48 hours beginning 15 minutes after the last bolus. Follow with an oral maintenance dose of 100 mg twice daily. In patients who do not tolerate the full IV dose start 25 to 50 mg PO within 15 minutes of the last IV dose. Dosage based on degree of intolerance. May have to discontinue metoprolol.
Treatment of atrial fibrillation (unlabeled):
2.5 to 5 mg as an IV injection over 2 to 5 minutes as necessary to control rate up to a total dose of 15 mg in a 10- to 15-minute period if indicated.
Treatment of ventricular rate control/hypertension (unlabeled):
1.25 to 5 mg every 6 to 12 hours. Begin with a lower initial dose and titrate to response. Up to 15 mg every 3 to 6 hours has been used.
Dose adjustments
See Drug/Lab Interactions. ■ Not required in impaired renal function. ■ In patients with impaired hepatic function, start at a low dose and titrate upward slowly.
Dilution
May be given undiluted.
Storage:
Store at CRT. Protect from light.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
One source suggests the following compatibilities:
Y-site:
Abciximab (ReoPro), alteplase (tPA), amiodarone (Nexterone), argatroban, bivalirudin (Angiomax), ceftaroline (Teflaro), diltiazem (Cardizem), eptifibatide (Integrilin), furosemide (Lasix), heparin, meperidine (Demerol), milrinone (Primacor), morphine, nesiritide (Natrecor), nitroprusside sodium (Nitropress), procainamide (Pronestyl).
Rate of administration
A single dose over 1 minute. Monitor ECG, HR, and BP and discontinue metoprolol if adverse symptoms occur (bradycardia less than 45 beats/min, heart block greater than first degree, systolic BP less than 100 mm Hg, or moderate to severe cardiac failure).
Actions
Metoprolol is a cardioselective (B1) adrenergic receptor blocker. Its mechanism of action in patients with suspected or definite myocardial infarction is not known, but its use has been shown to reduce the 3-month mortality rate in this patient population. It reduces the incidence of recurrent myocardial infarctions and reduces the size of the infarct and the incidence of fatal arrhythmias. Reduces HR, systolic BP, and cardiac output. Well distributed throughout the body, it acts within 1 to 2 minutes and lasts about 3 to 4 hours. Maximum beta blockade is achieved in approximately 20 minutes. Metabolized in the liver by the cytochrome P450 enzyme system, primarily CYP2D6. Excreted as metabolites in the urine. Crosses placental barrier. Secreted in breast milk.
Indications and uses
To reduce cardiac mortality in hemodynamically stable individuals with suspected or definite myocardial infarction (used in conjunction with oral metoprolol maintenance therapy). ■ Treatment of hypertension, angina pectoris, and CHF in oral dosage form.
Unlabeled uses:
Treatment of atrial fibrillation and unstable angina. ■ Has been used in the perioperative period to reduce cardiac morbidity and mortality in patients at risk. ■ Treatment of ventricular rate control and hypertension in patients who cannot take PO medications.
Contraindications
HR below 45 beats/min, second- or third-degree heart block, significant first-degree heart block (PR interval equal to or greater than 0.24 second), systolic BP below 100 mm Hg, or moderate to severe cardiac failure. ■ Hypersensitivity to metoprolol or to any of the excipients. Use caution in patients with hypersensitivity to other beta-blockers (e.g., atenolol [Tenormin], esmolol [Brevibloc], propranolol). Cross-sensitivity between beta-blockers can occur. ■ Severe peripheral arterial circulatory disorders or sick sinus syndrome in patients with angina or hypertension.
Precautions
Use caution in CHF. Beta blockade may depress myocardial contractility and precipitate or exacerbate heart failure and cardiogenic shock. ■ Use caution in presence of heart failure controlled by digoxin. Both drugs slow AV conduction. Bradycardia, sinus pause, heart block, and cardiac arrest have occurred. Patients with first-degree AV block, sinus node dysfunction, or conduction disorders may be at increased risk; see Antidote. ■ May produce significant first- (PR interval equal to or greater than 0.26 second), second-, or third-degree heart block. Acute MI can also cause heart block. ■ Metoprolol decreases sinus heart rate. MI may also produce significant lowering of HR; see Antidote. ■ Routine withdrawal of chronically administered beta-blockers before major surgery is not necessary; however, the risks of general anesthesia and surgical procedures may be increased by the impaired ability of the heart to respond to reflex adrenergic stimuli. ■ Use caution in patients with a history of severe anaphylactic reactions to a variety of allergens; they may be more reactive to repeated challenge (either accidental, diagnostic, or therapeutic) and may be unresponsive to the usual doses of epinephrine used to treat hypersensitivity reactions; see Drug/Lab Interactions. ■ May mask tachycardia occurring with hypoglycemia in diabetes and tachycardia of hyperthyroidism. ■ In general, patients with bronchospastic disease should not receive beta-blockers, including metoprolol. Because of its relative beta selectivity, metoprolol may be used with extreme caution in these patients. Monitor pulmonary function closely; see Antidote. ■ Use with caution in patients with impaired hepatic function; see Dose Adjustments. ■ May cause arrhythmia, angina, MI, or death if stopped abruptly (more of an issue with chronic oral therapy); see prescribing information. ■ May cause severe bradycardia in patients with Wolff-Parkinson-White syndrome. ■ Contraindicated in patients known to have or suspected of having a pheochromocytoma. If metoprolol is required, it should be given in combination with an alpha-blocker (e.g., phenoxybenzamine [Dibenzyline]) and only after the alpha-blocker has been initiated. ■ See Drug/Lab Interactions.
Monitor:
Continuous ECG, HR, and BP monitoring is mandatory with use of IV metoprolol. ■ Hemodynamic status must be closely monitored. If heart failure or hypotension occurs or persists despite appropriate treatment, metoprolol should be discontinued. Assess extent of myocardial damage. Invasive monitoring of central venous, pulmonary capillary wedge, and arterial pressure may be required. ■ See Drug/Lab Interactions and Antidote.
Patient education:
Report any breathing difficulty promptly.
Maternal/child:
Category C: safety for use in pregnancy and breast-feeding and in pediatric patients not established. ■ If a pregnancy occurs, women should inform their physician.
Elderly:
Age-related differences in safety and effectiveness not identified; however, greater sensitivity of some elderly cannot be ruled out. Dose with caution. ■ May exacerbate mental impairment.
Drug/lab interactions
Concurrent use with calcium channel blockers (e.g., diltiazem, verapamil) may potentiate both drugs and result in severe depression of myocardium and AV conduction and severe hypotension. ■ Concurrent use with antihypertensive agents, including alpha-adrenergic blockers (e.g., clonidine [Catapres], guanfacine [Tenex], methyldopa, reserpine), may result in excessive hypotension. Dose adjustment may be required. ■ Concurrent administration with hydralazine may decrease metabolism and increase concentrations of metoprolol. ■ Potent inhibitors of the CYP2D6 enzyme may increase plasma concentrations of metoprolol and decrease its cardioselectivity. These inhibitors include antidepressants (e.g., clomipramine [Anafranil], desipramine [Norpramin], fluoxetine [Prozac], fluvoxamine [Luvox], paroxetine [Paxil], sertraline [Zoloft], bupropion [Wellbutrin]), antipsychotics (e.g., chlorpromazine, fluphenazine, haloperidol [Haldol], thioridazine [Mellaril]), antiarrhythmics (e.g., quinidine, propafenone [Rythmol]), antiretrovirals (e.g., ritonavir [Norvir]), antihistamines (e.g., diphenhydramine [Benadryl]), antimalarials (e.g., hydroxychloroquine [Plaquenil], quinidine), allylamine antifungals (e.g., terbinafine [Lamisil]), and medications for stomach ulcers (e.g., cimetidine [Tagamet]). ■ Concurrent use within 14 days of MAO inhibitors (selegiline [Eldepryl]) may cause severe hypertension. ■ Use with sympathomimetic agents (e.g., epinephrine, norepinephrine, phenylephrine) or xanthines (e.g., aminophylline) may negate therapeutic effects of both drugs. ■ Effects of beta-adrenergic blocking agents may be decreased by anti-inflammatory drugs (e.g., NSAIDs), barbiturates, rifampin, salicylates, and others. ■ Inhalation anesthetics, phenytoin (Dilantin), and quinolone antibiotics (e.g., ciprofloxacin) may increase myocardial depressant effects and hypotension. ■ Beta-adrenergic blocking agents may be continued during the perioperative period in most patients; however, use caution with selected anesthetic agents that may depress the myocardium. ■ Potentiates effects of oral antidiabetics, catecholamine-depleting drugs (e.g., reserpine), insulin, lidocaine, and skeletal muscle relaxants; monitor carefully. Dose adjustment may be required. ■ Concurrent use with clonidine may precipitate acute hypertension if one or both agents are stopped abruptly. Withdraw metoprolol first. ■ Effects decreased when hypothyroid patient is converted to a euthyroid state; adjust dose as indicated. ■ Used concurrently with digoxin or alpha-adrenergic blockers (e.g., phentolamine [Regitine]) as indicated. ■ Use caution; both digoxin and beta-blockers (e.g., atenolol, esmolol, metoprolol, propranolol) slow AV conduction. May increase risk of bradycardia. ■ Patients taking beta-blockers who are exposed to a potential allergen may be unresponsive to the usual dose of epinephrine used to treat a hypersensitivity reaction. ■ May enhance the vasoconstrictive action of ergot alkaloids. ■ In general, withhold administration of a beta-blocker before dipyridamole testing; monitor HR carefully following dipyridamole injection.
Side effects
Abdominal pain, bradyarrhythmias, bronchospasm, cardiac failure, claudication, confusion, dizziness, dyspnea, elevated liver function tests, first-degree heart block, hallucinations, headache, hepatitis, hypotension, jaundice, nausea, nightmares, pruritus, rash, reduced libido, respiratory distress, second- or third-degree heart block, sleep disturbances, syncopal attacks, tiredness, unstable diabetes, vertigo, visual disturbances.
Antidote
For any side effect, discontinue drug and notify physician immediately. Patients with myocardial infarction may be more hemodynamically unstable; treat with caution. Use atropine (0.25 to 0.5 mg) for bradycardia or heart block; use isoproterenol with caution if atropine is not effective. Glucagon 5 to 10 mg IV may be effective if atropine and isoproterenol are not (investigational use). Transvenous cardiac pacing may be needed. Treat hypotension with IV fluids if indicated or vasopressors (dopamine or norepinephrine [Levarterenol]); treat cause of hypotension (e.g., bradycardia). Use all vasopressors with extreme caution; severe hypotension can result. Use digoxin and diuretics at first sign of cardiac failure; dobutamine, isoproterenol, or glucagon may be required. Use aminophylline or isoproterenol (with extreme care) for bronchospasm, and glucagon or IV glucose for hypoglycemia. Treat other side effects symptomatically; resuscitate as necessary.
Metronidazole hydrochloride 
(meh-troh-NYE-dah-zohl hy-droh-KLOR-eyed)
Flagyl IV, Flagyl IV RTU
Antibacterial
Antiprotozoal
Amebicide
pH 4.5 to 7
Usual dose
May transfer to oral therapy when condition warrants (usual PO dose is 7.5 mg/kg every 6 hours).
Anaerobic infections:
Begin with an initial loading dose of 15 mg/kg of body weight. Follow with 7.5 mg/kg (up to 1 Gm/dose) in 6 hours and every 6 hours thereafter for 7 to 10 days or longer if indicated. Do not exceed 4 Gm in 24 hours.
Surgical prophylaxis to prevent postoperative infection in contaminated or potentially contaminated colorectal surgery:
15 mg/kg infused over 30 to 60 minutes and completed 1 hour before surgery. Follow with 7.5 mg/kg over 30 to 60 minutes in 6 hours and in 12 hours.
Pediatric dose
Safety for use in infants and other pediatric patients not established, but is used for anaerobic infections; see Maternal/Child.
Anaerobic infections:
Pediatric patients more than 7 days of age:
7.5 mg/kg every 6 hours with a maximum dose of 4 Gm/24 hr.
Another source recommends age- and weight-specific doses as follows:
Less than 7 days of age weighing less than 1.2 kg:
7.5 mg/kg every 48 hours.
Less than 7 days of age weighing 1.2 to 2 kg:
7.5 mg/kg every 24 hours.
Less than 7 days of age weighing 2 or more kg:
7.5 mg/kg every 12 hours.
7 days of age or older weighing less than 1.2 kg:
7.5 mg/kg every 24 hours.
7 days of age or older weighing 1.2 to 2 kg:
7.5 mg/kg every 12 hours.
7 days of age or older weighing 2 or more kg:
15 mg/kg every 12 hours.
Dose adjustments
Reduce dose by 50% in patients with severe (Child-Pugh Class C) hepatic impairment. ■ Increase intervals in neonates; see Pediatric Dose. ■ No dose adjustment is indicated in mild to moderate impaired renal function. Recommendations vary for patients with a CrCl of less than 10 mL/min who are not on dialysis; consider reducing dose by 50% or increasing the interval to every 12 hours. ■ Dose adjustment not indicated in anuric patients; accumulated metabolites readily removed by dialysis. 40% to 65% of a metronidazole dose can be removed by dialysis depending on length of dialysis session and type of dialyzer membrane used. If metronidazole administration cannot be separated from dialysis session, consider supplemental dose following dialysis. ■ Continuous NG suction may remove sufficient metronidazole in gastric aspirate to reduce serum levels. No dose adjustment is recommended.
Dilution
All solutions are prediluted and ready to use (5 mg/mL) except Flagyl IV. The powder form (Flagyl IV) is not readily available but requires a specific dilution process. Initially add 4.4 mL SWFI or NS for injection (with or without preservative) to provide a solution with an approximate concentration of 100 mg/mL (500 mg in 5 mL). Solution must be clear. Solution will be yellow to yellow-green in color with a pH of 0.5 to 2. The desired dose of properly reconstituted solution may be further diluted with NS, D5W, or LR. Do not exceed a concentration of 8 mg/mL (500 mg in 100 mL yields a concentration of 5 mg/mL). Must be neutralized before infusion with 5 mEq of sodium bicarbonate per 500 mg metronidazole to achieve an approximate pH of 6 to 7. Mix thoroughly. CO2 gas will be generated and may require venting. Do not use plastic containers in series connections. Risk of air embolism is present. Avoid all contact with aluminum in needles and syringes in all situations.
Storage:
Store at room temperature (25° C [75° F]). Do not refrigerate. Protect from light. Do not remove premixed product from overwrap until ready for use. Reconstituted vial is stable for 96 hours when stored below 86° F (30° C ) in room light. Diluted, neutralized solution must be used within 24 hours of mixing. Do not refrigerate neutralized solution; precipitation may occur.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer recommends, “Administer separately, discontinue the primary IV during administration, and do not introduce additives into the solution.” Do not use equipment containing aluminum.
One source suggests the following compatibilities:
Additive:
Not recommended by manufacturer.
Ampicillin, cefazolin (Ancef), cefepime (Maxipime), cefotaxime (Claforan), cefoxitin (Mefoxin), ceftazidime (Fortaz), ceftriaxone (Rocephin), cefuroxime (Zinacef), ciprofloxacin (Cipro IV), fluconazole (Diflucan), gentamicin, hydrocortisone sodium succinate (Solu-Cortef), midazolam (Versed), penicillin G potassium, tobramycin.
Y-site:
Acyclovir (Zovirax), allopurinol (Aloprim), amifostine (Ethyol), anidulafungin (Eraxis), bivalirudin (Angiomax), caspofungin (Cancidas), ceftaroline (Teflaro), cisatracurium (Nimbex), cyclophosphamide (Cytoxan), dexmedetomidine (Precedex), diltiazem (Cardizem), dimenhydrinate, docetaxel (Taxotere), dopamine, doripenem (Doribax), doxapram (Dopram), doxorubicin liposomal (Doxil), enalaprilat (Vasotec IV), esmolol (Brevibloc), etoposide phosphate (Etopophos), fenoldopam (Corlopam), fluconazole (Diflucan), foscarnet (Foscavir), gemcitabine (Gemzar), granisetron (Kytril), heparin, hetastarch in electrolytes (Hextend), hydromorphone (Dilaudid), 6% hydroxyethyl starch (Voluven), labetalol, linezolid (Zyvox), lorazepam (Ativan), magnesium sulfate, melphalan (Alkeran), meperidine (Demerol), methylprednisolone (Solu-Medrol), midazolam (Versed), milrinone (Primacor), morphine, nicardipine (Cardene IV), palonosetron (Aloxi), piperacillin/tazobactam (Zosyn), remifentanil (Ultiva), sargramostim (Leukine), tacrolimus (Prograf), teniposide (Vumon), theophylline, thiotepa, vasopressin, vinorelbine (Navelbine).
Rate of administration
Must be given as a slow intermittent or continuous IV infusion, each single dose evenly distributed over 1 hour. Discontinue primary IV during administration.
Surgical prophylaxis:
Administer each single dose over 30 to 60 minutes.
Actions
A bactericidal agent with cytotoxic effects, active against specific obligate anaerobic bacteria and protozoa. Does not possess any clinically relevant activity against facultative anaerobes or obligate aerobes. Metronidazole enters the organism by passive diffusion and is reduced. The reduced form and free radicals that are produced during the reduction reaction interact with DNA, leading to inhibition of DNA synthesis and to DNA degradation and death of bacteria. The precise mechanism of action is unclear. Metronidazole is widely distributed. Plasma concentrations are directly proportional to dose given. Onset of action is prompt. Metabolized in the liver. Half-life is 8 hours. Crosses placental and blood-brain barriers. Excreted primarily in urine, some in feces. Secreted in breast milk.
Indications and uses
Treatment of serious infections caused by susceptible strains of anaerobic bacteria, including serious intra-abdominal, skin and skin structure, gynecologic, bone and joint, CNS, and lower respiratory tract infections, bacterial septicemia, and endocarditis. Is effective in Bacteroides fragilis infections resistant to clindamycin, chloramphenicol, and penicillin. ■ Perioperative prophylaxis to reduce infection rates in contaminated or potentially contaminated colorectal surgery. ■ Given orally for amebiasis, giardiasis, Helicobacter pylori eradication, and other indications.
Unlabeled uses:
Clostridium difficile–associated diarrhea (CDAD), Crohn’s disease, pelvic inflammatory disease.
Contraindications
Hypersensitivity to metronidazole or other nitroimidazole derivatives; use of disulfiram within the last 2 weeks; use of alcohol or products containing propylene glycol during and for at least 3 days after therapy with metronidazole.
Precautions
A mixed (anaerobic/aerobic) infection will require use of additional antibiotics targeted for treatment of the aerobic infection. ■ Sensitivity studies indicated to determine susceptibility of the causative organism to metronidazole. ■ To reduce the development of drug-resistant bacteria and maintain its effectiveness, metronidazole should be used to treat or prevent only those infections proven or strongly suspected to be caused by bacteria. ■ Avoid prolonged use of the drug; superinfection caused by overgrowth of nonsusceptible organisms may result. ■ Symptoms of candidiasis may be exacerbated and require treatment. ■ Encephalopathy has been reported in association with cerebellar toxicity characterized by ataxia, dizziness, and dysarthria. CNS lesions have been seen on MRI. Generally reversible within days to weeks after metronidazole is discontinued. ■ Optic neuropathy and peripheral neuropathy (mainly sensory with S/S of numbness or paresthesia of extremities) have been reported. ■ May cause seizures. ■ Aseptic meningitis has been reported. Symptoms may occur within hours of dose administration and generally resolve after metronidazole is discontinued. ■ Use caution in patients predisposed to edema and/or taking corticosteroids, in patients with impaired cardiac function (contains 27 to 28 mEq sodium/Gm), CNS disease, hepatic or renal impairment, or a history of blood dyscrasias. ■ Clostridium difficile–associated diarrhea (CDAD) has been reported. May range from mild diarrhea to fatal colitis. Consider in patients who present with diarrhea during or after treatment with metronidazole. ■ Carcinogenic in rodents; avoid unnecessary use and restrict use to approved indications.
Monitor:
Rotate IV site frequently to avoid thrombophlebitis. Avoid extravasation. ■ Mild leukopenia has been reported. Obtain total and differential leukocyte counts before, during, and after prolonged or repeated courses of therapy. ■ Monitor for S/S of toxicity in patients with hepatic or renal impairment and in the elderly. ■ Monitor for neurologic S/S (e.g., ataxia, dizziness, dysarthrias, numbness, paresthesia, and seizures); see Antidote. ■ Transfer to oral dosing as soon as practical. ■ See Drug/Lab Interactions.
Patient education:
Avoid alcohol, alcohol-containing preparations, and disulfiram; toxic reactions will occur. ■ Promptly report any neurologic side effects (e.g., seizures, numbness, or paresthesia of an extremity). ■ Promptly report diarrhea or bloody stools that occur during treatment or up to several months after an antibiotic has been discontinued; may indicate CDAD and require treatment.
Maternal/child:
Category B: use during pregnancy only if clearly needed. ■ Discontinue breast-feeding during metronidazole therapy and for 24 hours after therapy ends. ■ Safety for use in pediatric patients and neonates not established. The elimination half-life, measured during the first 3 days of life, was inversely related to gestational age. Half-life markedly extended in newborns; adjust intervals; see Pediatric Dose.
Elderly:
Pharmacokinetics altered in the elderly; monitor for metronidazole-associated adverse events and adjust dose accordingly.
Drug/lab interactions
Avoid alcohol and alcohol-containing preparations for at least 3 days after taking any dose of metronidazole; a disulfiram-like reaction (abdominal cramps, flushing, headaches, nausea and vomiting) may occur. ■ Avoid administration of metronidazole to patients who have taken disulfiram within the last 2 weeks. Psychotic reactions have been reported. ■ Concurrent use with drugs that induce microsomal enzyme activity (e.g., phenobarbital, phenytoin [Dilantin]) may increase metabolism of metronidazole and decrease plasma levels. ■ Administration with drugs that inhibit microsomal liver enzyme activity (e.g., cimetidine [Tagamet]) may prolong the half-life of metronidazole and increase metronidazole plasma levels. ■ May decrease clearance and increase serum concentration of phenytoin. ■ May decrease metabolism and increase anticoagulant effects of warfarin (Coumadin). Monitor PT/INR periodically. ■ May increase lithium levels and cause toxicity. ■ May increase plasma concentrations of busulfan, increasing the risk of serious busulfan toxicity. Avoid concomitant use if possible. If concomitant administration is medically necessary, monitor busulfan plasma concentration and adjust busulfan dose accordingly. ■ May interfere with selected chemistry studies (e.g., AST, ALT, LDH, triglycerides, glucose hexokinase).
Side effects
The most serious side effects include aseptic meningitis, convulsive seizures, encephalopathy, and optic and peripheral neuropathy. Abdominal cramping; anorexia; ataxia; CDAD; confusion; constipation; cystitis; darkened deep red urine; decreased libido; depression; diarrhea; dizziness; dryness of the mouth, vagina, or vulva; dysarthria; dysuria; epigastric distress; fever; fleeting joint pain; flushing; furry tongue; glossitis; headache; incontinence; insomnia; irritability; metallic taste (expected); nasal congestion; nausea; neutropenia (reversible); numbness; painful coitus; pancreatitis; paresthesia; pelvic pressure; polyuria; proctitis; pruritus; psychosis; rash; Stevens-Johnson syndrome; stomatitis; syncope; thrombocytopenia (reversible); thrombophlebitis; toxic epidermal necrolysis; T-wave flattening; urticaria; vomiting; and weakness have occurred.
Antidote
Notify physician of all side effects. Treatment will be symptomatic and supportive. Evaluate risk versus benefit of continuing therapy in patients who develop abnormal neurologic S/S (e.g., encephalopathy, seizures, or signs of peripheral neuropathy). Treat CDAD with fluids, electrolytes, protein supplements, and oral vancomycin (Vancocin) or metronidazole (Flagyl) as indicated. In severe cases, surgical evaluation may be indicated. Removed by hemodialysis. Treat anaphylaxis and resuscitate as necessary.
Micafungin sodium
(my-kah-FUN-gin SO-dee-um)
Mycamine
Antifungal (echinocandin)
pH 5 to 7
Usual dose
Treatment of candidemia, acute disseminated candidiasis, candida peritonitis and abscesses:
100 mg/day as an infusion. Mean duration of treatment during clinical studies was 15 days (range 10 to 47 days).
Treatment of esophageal candidiasis:
150 mg/day as an infusion. Mean duration of treatment during clinical studies was 15 days (range 10 to 30 days).
Prophylaxis of candida infections in hematopoietic stem cell transplant (HSCT) recipients:
50 mg/day as an infusion. Mean duration of treatment in patients who responded successfully during clinical studies was 19 days (range 6 to 51 days).
Pediatric dose
Recommended doses for pediatric patients based on indication and weight are outlined in the following chart.
| Micafungin Dosage in Pediatric Patients 4 Months of Age or Older | ||
| Indication | Pediatric Dose Given Once Daily | |
| 30 kg or less | Greater than 30 kg | |
| Treatment of candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses | 2 mg/kg (maximum daily dose 100 mg) | |
| Treatment of esophageal candidiasis | 3 mg/kg | 2.5 mg/kg (maximum daily dose 150 mg) |
| Prophylaxis of Candida infections in HSCT recipients | 1 mg/kg (maximum daily dose 50 mg) | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


