CHAPTER 13 Judith Haber and Geri LoBiondo-Wood After reading this chapter, you should be able to do the following: • Describe the historical background that led to the development of ethical guidelines for the use of human subjects in research. • Identify the essential elements of an informed consent form. • Evaluate the adequacy of an informed consent form. • Describe the institutional review board’s role in the research review process. • Identify populations of subjects who require special legal and ethical research considerations. • Appreciate the nurse researcher’s obligations to conduct and report research in an ethical manner. • Describe the nurse’s role as patient advocate in research situations. Go to Evolve at http://evolve.elsevier.com/LoBiondo/ for review questions, critiquing exercises, and additional research articles for practice in reviewing and critiquing. Nurses are in an ideal position to promote patients’ awareness of the role played by research in the advancement of science and improvement in patient care. Embedded in our professional Code of Ethics (American Nurses Association [ANA], 2001) is the charge to protect patients from harm. Code of ethics not only state the rules and regulations regarding the involvement of human research subjects to ensure that research is conducted legally and ethically, but also address appropriate conduct of researchers governed by those rules. Researchers themselves and caregivers providing care to patients, who also happen to be research subjects, must be fully committed to the tenets of informed consent and patients’ rights. The principle “the ends justify the means” must never be tolerated. Researchers and caregivers of research subjects must take every precaution to protect those being studied from physical or mental harm or discomfort. It is not always clear what constitutes harm or discomfort. The Nuremberg Code and its definitions of the terms voluntary, legal capacity, sufficient understanding, and enlightened decision have been the subject of numerous court cases and presidential commissions involved in setting ethical standards in research (Amdur & Bankert, 2011). The code that was developed requires informed consent in all cases but makes no provisions for any special treatment of children, the elderly, or the mentally incompetent. Several other international standards have followed, the most notable of which was the Declaration of Helsinki, which was adopted in 1964 by the World Medical Assembly and later revised in 1975. In the United States, federal guidelines for the ethical conduct of research involving human subjects were not developed until the 1970s. Despite the supposed safeguards provided by the federal guidelines, some of the most atrocious, and hence memorable, examples of unethical research studies took place in the United States as recently as the 1990s. These examples are highlighted in Table 13-1. They are sad reminders of our own tarnished research heritage and illustrate the human consequences of not adhering to ethical research standards. TABLE 13-1 HIGHLIGHTS OF UNETHICAL RESEARCH STUDIES CONDUCTED IN THE UNITED STATES The conduct of harmful, illegal research made additional controls necessary. In 1973 the U.S. Department of Health, Education, and Welfare published the first set of proposed regulations on the protection of human subjects. The most important provision was a regulation mandating that an institutional review board functioning in accordance with specifications of the department must review and approve all studies. The National Research Act, passed in 1974 (Public Law 93-348), created the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. A major charge of the Commission was to identify the basic principles that should underlie the conduct of biomedical and behavioral research involving human subjects and to develop guidelines to ensure that research is conducted in accordance with those principles (Amdur & Bankert, 2011). Three ethical principles were identified as relevant to the conduct of research involving human subjects: the principles of respect for persons, beneficence, and justice. They are defined in Box 13-1. Included in a report issued in 1979, called the Belmont Report, these principles provided the basis for regulations affecting research sponsored by the federal government. The Belmont Report also served as a model for many of the ethical codes developed by scientific disciplines (National Commission, 1978). In 1980, the U.S. Department of Health and Human Services (DHHS) developed a set of regulations in response to the Commission’s recommendations. These regulations were published in 1981 and have been revised several times (DHHS, 2009). They include • General requirements for informed consent • Documentation of informed consent • IRB review of research proposals • Exempt and expedited review procedures for certain kinds of research 1. Right to self-determination 2. Right to privacy and dignity 3. Right to anonymity and confidentiality These rights apply to all involved in research, including research team members who may be involved in data collection, practicing nurses involved in the research setting, and subjects participating in the study. As you read a research article, you must realize that any issues highlighted in Table 13-2 should have been addressed and resolved before a research study is approved for implementation. TABLE 13-2 • Coercion occurs when an overt threat of harm or excessive reward is presented to ensure compliance. • Covert data collection occurs when people become research subjects and are exposed to research treatments without their knowledge. • Deception occurs when subjects are actually misinformed about the purpose of the research. • Potential for violation of the right to self-determination is greater for subjects with diminished autonomy; they have decreased ability to give informed consent and are vulnerable.
Legal and ethical issues
Ethical and legal considerations in research: A historical perspective
Past ethical dilemmas in research
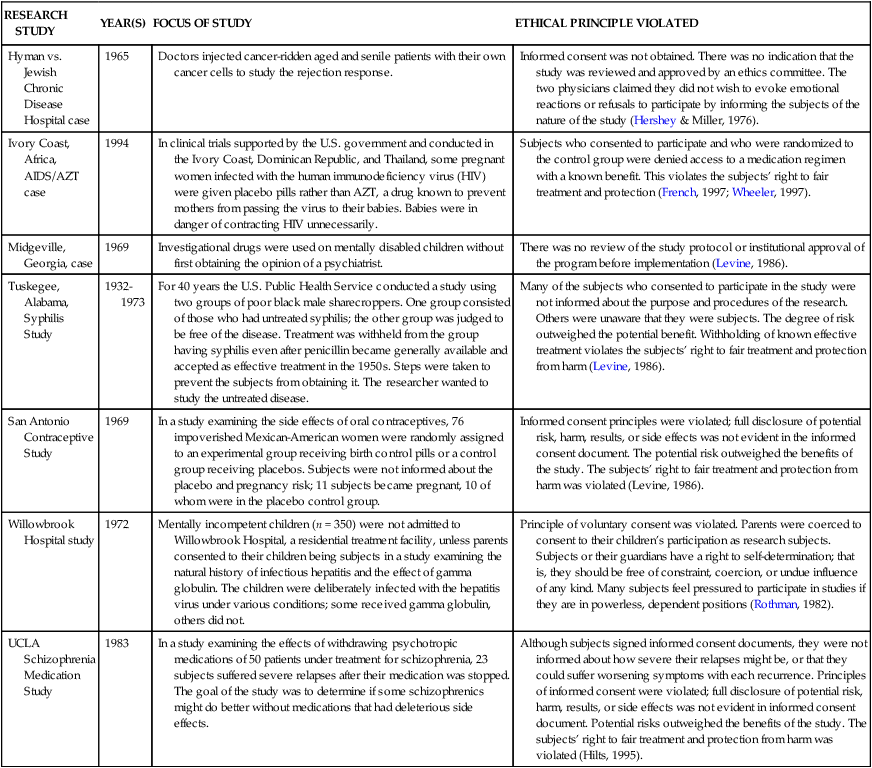
RESEARCH STUDY
YEAR(S)
FOCUS OF STUDY
ETHICAL PRINCIPLE VIOLATED
Hyman vs. Jewish Chronic Disease Hospital case
1965
Doctors injected cancer-ridden aged and senile patients with their own cancer cells to study the rejection response.
Informed consent was not obtained. There was no indication that the study was reviewed and approved by an ethics committee. The two physicians claimed they did not wish to evoke emotional reactions or refusals to participate by informing the subjects of the nature of the study (Hershey & Miller, 1976).
Ivory Coast, Africa, AIDS/AZT case
1994
In clinical trials supported by the U.S. government and conducted in the Ivory Coast, Dominican Republic, and Thailand, some pregnant women infected with the human immunodeficiency virus (HIV) were given placebo pills rather than AZT, a drug known to prevent mothers from passing the virus to their babies. Babies were in danger of contracting HIV unnecessarily.
Subjects who consented to participate and who were randomized to the control group were denied access to a medication regimen with a known benefit. This violates the subjects’ right to fair treatment and protection (French, 1997; Wheeler, 1997).
Midgeville, Georgia, case
1969
Investigational drugs were used on mentally disabled children without first obtaining the opinion of a psychiatrist.
There was no review of the study protocol or institutional approval of the program before implementation (Levine, 1986).
Tuskegee, Alabama, Syphilis Study
1932-1973
For 40 years the U.S. Public Health Service conducted a study using two groups of poor black male sharecroppers. One group consisted of those who had untreated syphilis; the other group was judged to be free of the disease. Treatment was withheld from the group having syphilis even after penicillin became generally available and accepted as effective treatment in the 1950s. Steps were taken to prevent the subjects from obtaining it. The researcher wanted to study the untreated disease.
Many of the subjects who consented to participate in the study were not informed about the purpose and procedures of the research. Others were unaware that they were subjects. The degree of risk outweighed the potential benefit. Withholding of known effective treatment violates the subjects’ right to fair treatment and protection from harm (Levine, 1986).
San Antonio Contraceptive Study
1969
In a study examining the side effects of oral contraceptives, 76 impoverished Mexican-American women were randomly assigned to an experimental group receiving birth control pills or a control group receiving placebos. Subjects were not informed about the placebo and pregnancy risk; 11 subjects became pregnant, 10 of whom were in the placebo control group.
Informed consent principles were violated; full disclosure of potential risk, harm, results, or side effects was not evident in the informed consent document. The potential risk outweighed the benefits of the study. The subjects’ right to fair treatment and protection from harm was violated (Levine, 1986).
Willowbrook Hospital study
1972
Mentally incompetent children (n = 350) were not admitted to Willowbrook Hospital, a residential treatment facility, unless parents consented to their children being subjects in a study examining the natural history of infectious hepatitis and the effect of gamma globulin. The children were deliberately infected with the hepatitis virus under various conditions; some received gamma globulin, others did not.
Principle of voluntary consent was violated. Parents were coerced to consent to their children’s participation as research subjects. Subjects or their guardians have a right to self-determination; that is, they should be free of constraint, coercion, or undue influence of any kind. Many subjects feel pressured to participate in studies if they are in powerless, dependent positions (Rothman, 1982).
UCLA Schizophrenia Medication Study
1983
In a study examining the effects of withdrawing psychotropic medications of 50 patients under treatment for schizophrenia, 23 subjects suffered severe relapses after their medication was stopped. The goal of the study was to determine if some schizophrenics might do better without medications that had deleterious side effects.
Although subjects signed informed consent documents, they were not informed about how severe their relapses might be, or that they could suffer worsening symptoms with each recurrence. Principles of informed consent were violated; full disclosure of potential risk, harm, results, or side effects was not evident in informed consent document. Potential risks outweighed the benefits of the study. The subjects’ right to fair treatment and protection from harm was violated (Hilts, 1995).
Protection of human rights
DEFINITION
VIOLATION OF BASIC HUMAN RIGHT
EXAMPLE
RIGHT TO SELF-DETERMINATION
Based on the principle of respect for persons, people should be treated as autonomous agents who have the freedom to choose without external controls. An autonomous agent is one who is informed about a proposed study and allowed to choose to participate or not to participate; subjects have the right to withdraw from a study without penalty. Subjects with diminished autonomy are entitled to protection. They are more vulnerable because of age, legal or mental incompetence, terminal illness, or confinement to an institution. Justification for use of vulnerable subjects must be provided.
A subject’s right to self-determination is violated through use of coercion, covert data collection, and deception.
Subjects may feel that their care will be adversely affected if they refuse to participate in research. The Jewish Chronic Disease Hospital Study (see Table 13-1) is an example in which patients and their doctors did not know that cancer cells were being injected. In the Milgrim (1963) study, subjects were deceived when asked to administer electric shocks to another person; the person was really an actor who pretended to feel the shocks. Subjects administering the shocks were very stressed by participating in this study, although they were not administering shocks at all. The Willowbrook Study (see Table 13-1) is an example of how coercion was used to obtain parental consent of vulnerable mentally retarded children who would not be admitted to the institution unless the children participated in a study in which they were deliberately injected with the hepatitis virus.
RIGHT TO PRIVACY AND DIGNITY
Based on the principle of respect, privacy is the freedom of a person to determine the time, extent, and circumstances under which private information is shared or withheld from others.
The Privacy Act of 1974 was instituted to protect subjects from such violations. These occur most frequently during data collection when invasive questions are asked that might result in loss of job, friendships, or dignity; or might create embarrassment and mental distress. It also may occur when subjects are unaware that information is being shared with others.
Subjects may be asked personal questions such as the following: “Were you sexually abused as a child?”“Do you use drugs?”“What are your sexual preferences?” When questions are asked using hidden microphones or hidden tape recorders, the subjects’ privacy is invaded because they have no knowledge that the data are being shared with others. Subjects also have a right to control access of others to their records.
RIGHT TO ANONYMITY AND CONFIDENTIALITY
Based on the principle of respect, anonymity exists when a subject’s identity cannot be linked, even by the researcher, with their individual responses.
Anonymity is violated when the subjects’ responses can be linked with their identity.
Subjects are given a code number instead of using names for identification purposes. Subjects’ names are never used when reporting findings.
Confidentiality means that individual identities of subjects will not be linked to the information they provide and will not be publicly divulged.
Confidentiality is breached when a researcher, either by accident or by direct action, allows an unauthorized person to gain access to study data that contains subjects’ identity information or responses that create a potentially harmful situation for subjects.
Breaches of confidentiality with regard to sexual preference, income, drug use, prejudice, or personality variables can be harmful to subjects. Data are analyzed as group data so individuals cannot be identified by their responses.
RIGHT TO FAIR TREATMENT
Based on the principle of justice, people should be treated fairly and receive what they are due or owed. Fair treatment is equitable subject selection and treatment during a study including: selection of subjects for reasons directly related to the problem studied vs. convenience, compromised position, or vulnerability. Also included is fair treatment of subjects during a study, including fair distribution of risks and benefits regardless of age, race, or socioeconomic status.
Injustices with regard to subject selection have occurred as a result of social, cultural, racial, and gender biases in society.
Historically, research subjects often have been obtained from groups of people who were regarded as having less “social value,” such as the poor, prisoners, slaves, the mentally incompetent, and the dying. Often subjects were treated carelessly, without consideration of physical or psychological harm.
The Tuskegee Syphilis Study (1973), the Jewish Chronic Disease Study (1965), the San Antonio Contraceptive Study (1969), and the Willowbrook Study (1972) (see Table 13-1) all provide examples related to unfair subject selection.
Investigators should not be late for data collection appointments, should terminate data collection on time, should not change agreed-on procedures or activities without consent, and should provide agreed-on benefits such as a copy of the study findings or a participation fee.
RIGHT TO PROTECTION FROM DISCOMFORT AND HARM
Based on the principle of beneficence, people must take an active role in promoting good and preventing harm in the world around them, as well as in research studies.
Discomfort and harm can be physical, psychological, social, or economic in nature.
There are five categories of studies based on levels of harm and discomfort:
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

Legal and ethical issues
Get Clinical Tree app for offline access