Titrate the infusion dose between 0.375 mcg/kg/min to 0.75 mcg/kg/min (26 mcg/min to 52 mcg/min for a 70-kg person) based on hemodynamic and clinical response. Do not exceed a total dose of 1.13 mg/kg/24 hr. Duration of infusion usually does not exceed 48 hours.
Dose adjustments
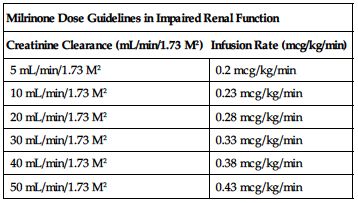
Reduced dose required in impaired renal function based on CrCl according to the following chart.
| Milrinone Dose Guidelines in Impaired Renal Function | |
| Creatinine Clearance (mL/min/1.73 M2) | Infusion Rate (mcg/kg/min) |
| 5 mL/min/1.73 M2 | 0.2 mcg/kg/min |
| 10 mL/min/1.73 M2 | 0.23 mcg/kg/min |
| 20 mL/min/1.73 M2 | 0.28 mcg/kg/min |
| 30 mL/min/1.73 M2 | 0.33 mcg/kg/min |
| 40 mL/min/1.73 M2 | 0.38 mcg/kg/min |
| 50 mL/min/1.73 M2 | 0.43 mcg/kg/min |
Dilution
Loading dose:
May be given undiluted, or each 1 mg (1 mL) may be diluted in 1 mL NS or 1/2NS for injection. Alternately, the loading dose may be diluted with NS, 1/2NS, or D5W to a total volume of 10 or 20 mL for injection.
Infusion:
Dilute with NS, 1/2NS, or D5W. Available prediluted as 200 mcg/mL in D5W. Amount of diluent may be increased or decreased based on patient fluid requirements. Another source suggests dilution with LR.
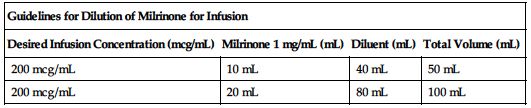
| Guidelines for Dilution of Milrinone for Infusion | |||
| Desired Infusion Concentration (mcg/mL) | Milrinone 1 mg/mL (mL) | Diluent (mL) | Total Volume (mL) |
| 200 mcg/mL | 10 mL | 40 mL | 50 mL |
| 200 mcg/mL | 20 mL | 80 mL | 100 mL |
May be given through Y-tube or three-way stopcock of IV infusion set but should never come in contact with furosemide (Lasix). Use only freshly prepared solutions.
Filters:
No data available from manufacturer.
Storage:
Store at room temperature before dilution; avoid freezing.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer states, “Do not add supplementary medications.” Forms an immediate precipitate with furosemide (Lasix).
One source suggests the following compatibilities:
Additive:
Not recommended by manufacturer. Quinidine gluconate.
Y-site:
Acyclovir (Zovirax), amikacin, amiodarone (Nexterone), ampicillin, argatroban, atracurium (Tracrium), bivalirudin (Angiomax), bumetanide, calcium chloride, calcium gluconate, caspofungin (Cancidas), cefazolin (Ancef), cefepime (Maxipime), cefotaxime (Claforan), ceftaroline (Teflaro), ceftazidime (Fortaz), cefuroxime (Zinacef), ciprofloxacin (Cipro IV), clindamycin (Cleocin), dexamethasone (Decadron), dexmedetomidine (Precedex), digoxin (Lanoxin), diltiazem (Cardizem), dobutamine, dopamine, doripenem (Doribax), epinephrine (Adrenalin), fenoldopam (Corlopam), fentanyl, gentamicin, heparin, hetastarch in electrolytes (Hextend), hydromorphone (Dilaudid), insulin (regular), isoproterenol (Isuprel), labetalol, lorazepam (Ativan), magnesium sulfate, meropenem (Merrem IV), methylprednisolone (Solu-Medrol), metoprolol (Lopressor), metronidazole (Flagyl IV), micafungin (Mycamine), midazolam (Versed), morphine, nesiritide (Natrecor), nicardipine (Cardene IV), nitroglycerin IV, nitroprusside sodium, norepinephrine (Levophed), oxacillin (Bactocill), pancuronium, piperacillin/tazobactam (Zosyn), potassium chloride (KCl), propofol (Diprivan), propranolol, quinidine gluconate, ranitidine (Zantac), rocuronium (Zemuron), sodium bicarbonate, telavancin (Vibativ), theophylline, ticarcillin/clavulanate (Timentin), tobramycin, torsemide (Demadex), vancomycin, vasopressin, vecuronium, verapamil.
Rate of administration
Loading dose:
A single dose evenly distributed over 10 minutes.
Infusion:
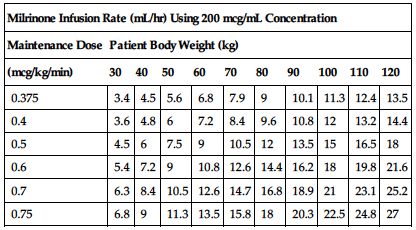
Use an infusion pump to deliver milrinone in recommended doses. The following manufacturer’s dose chart defines selected dose in mcg/kg/min in infusion rate of mL/hr. Adjust as indicated by physician’s orders and progress in patient’s condition. Reduce rate or stop infusion for excessive drop in BP.
| Milrinone Infusion Rate (mL/hr) Using 200 mcg/mL Concentration | ||||||||||
| Maintenance Dose | Patient Body Weight (kg) | |||||||||
| (mcg/kg/min) | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 |
| 0.375 | 3.4 | 4.5 | 5.6 | 6.8 | 7.9 | 9 | 10.1 | 11.3 | 12.4 | 13.5 |
| 0.4 | 3.6 | 4.8 | 6 | 7.2 | 8.4 | 9.6 | 10.8 | 12 | 13.2 | 14.4 |
| 0.5 | 4.5 | 6 | 7.5 | 9 | 10.5 | 12 | 13.5 | 15 | 16.5 | 18 |
| 0.6 | 5.4 | 7.2 | 9 | 10.8 | 12.6 | 14.4 | 16.2 | 18 | 19.8 | 21.6 |
| 0.7 | 6.3 | 8.4 | 10.5 | 12.6 | 14.7 | 16.8 | 18.9 | 21 | 23.1 | 25.2 |
| 0.75 | 6.8 | 9 | 11.3 | 13.5 | 15.8 | 18 | 20.3 | 22.5 | 24.8 | 27 |
Actions
A class of cardiac inotropic agent different in chemical structure and mode of action from digitalis glycosides and catecholamines. Similar to inamrinone, with fewer side effects. With a loading dose, peak effect occurs within 10 minutes. Continuous administration is required to maintain serum levels. It has positive inotropic action with vasodilator activity. Reduces afterload and preload by direct relaxant effect on vascular smooth muscle. Produces slight enhancement of AV node conduction. Cardiac output is improved without significant increases in HR or myocardial oxygen consumption or changes in arteriovenous oxygen difference. Pulmonary capillary wedge pressure, total peripheral resistance, diastolic BP, and mean arterial pressure are decreased. HR generally remains the same. Mean half-life is 2.4 hours. Primary route of excretion is in urine.
Indications and uses
Short-term management of patients with acute decompensated heart failure.
Contraindications
Hypersensitivity to milrinone or inamrinone.
Precautions
Not shown to be safe or effective for use longer than 48 hours. No improvement in symptoms and an increased risk of death have been reported. ■ Use caution in impaired renal function; serum levels may increase considerably. ■ May be given to digitalized patients without causing signs of digoxin toxicity; correct hypokalemia with potassium supplements. ■ May increase ventricular response in atrial flutter/fibrillation. Consider pretreatment with digoxin. ■ Additional fluids and electrolytes may be required to facilitate appropriate response in patients who have been vigorously diuresed and may have insufficient cardiac filling pressure. Use caution. ■ Safety for use in the acute phase of myocardial infarction not established. ■ Should not be used in patients with severe obstructive aortic or pulmonary valvular disease in lieu of surgical relief of the obstruction. May aggravate outflow tract obstruction in hypertrophic subaortic stenosis.
Monitor:
Observe closely. Continuous ECG monitoring required to allow for prompt detection and management of cardiac events, including life-threatening ventricular arrhythmias. Emergency equipment must be readily available. ■ Monitoring of BP, urine output, renal function, fluid and electrolyte changes (especially potassium), liver function tests, and body weight is recommended. ■ Monitoring of cardiac index, pulmonary capillary wedge pressure, central venous pressure, and plasma concentration is very useful. ■ Observe for orthopnea, dyspnea, and fatigue. ■ Reduce rate or stop infusion for excessive drop in BP. ■ As cardiac output and diuresis improve, a reduction in diuretic dose may be indicated. ■ Possible risk of arrhythmias. Risk further increased with excessive diuresis and/or hypokalemia. Replace potassium as indicated. ■ Infusion site reactions may occur. Monitor site carefully.
Maternal/child:
Category C: safety for use during pregnancy, breast-feeding, and in pediatric patients not established. Use during pregnancy only if potential benefit justifies potential risk.
Elderly:
Consider impaired renal function; may require a reduced dose.
Drug/lab interactions
Theoretical potential for interaction with calcium channel blockers (e.g., verapamil); no clinical evidence to date. ■ May cause additive hypotensive effects with any drug that produces hypotension (e.g., alcohol, benzodiazepines [e.g., diazepam, midazolam], lidocaine, paclitaxel). ■ No untoward drug interactions observed when used in a limited number of patients concurrently with captopril, chlorthalidone (Hygroton), diazepam (Valium), digoxin (Lanoxin), furosemide (Lasix), heparin, hydralazine, hydrochlorothiazide, insulin, isosorbide dinitrate (Sorbitrate), lidocaine, nitroglycerin, prazosin (Minipress), quinidine, spironolactone (Aldactone), warfarin (Coumadin), and potassium supplements. ■ See Monitor.
Side effects
Supraventricular and ventricular arrhythmias including nonsustained ventricular tachycardia do occur; rare cases of torsades de pointes have been reported. Abnormal liver function tests, anaphylactic shock (rare), angina, bronchospasm, chest pain, headaches, hypokalemia, hypotension, infusion site reactions, rash, and tremor have been reported.
Antidote
Notify the physician of any side effect. Based on degree of severity and condition of the patient, may be treated symptomatically, and dose may remain the same, be decreased, or the milrinone may be discontinued. Reduce rate or discontinue the drug at the first sign of marked hypotension and notify the physician. May be resolved by these measures alone or vasopressors (e.g., dopamine) may be required. Treat dysrhythmias with the appropriate drug. Resuscitate as necessary.
Mitomycin 
(my-toe-MY-sin)
MTC, Mutamycin
Antineoplastic (antibiotic)
pH 6 to 8
Usual dose
10 to 20 mg/M2 as a single dose. May be repeated in 6 to 8 weeks after adequate bone marrow recovery; see Dose Adjustments. Discontinue drug if no response after two courses of treatment.
Dose adjustments
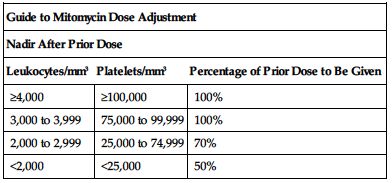
Subsequent doses based on posttreatment leukocyte and platelet counts. Withhold dose for leukocytes below 4,000/mm3 or platelet count below 100,000/mm3. Adjust subsequent doses based on nadir after the prior dose according to the following chart. ■ Lower usual dose range is indicated when used with other antineoplastic drugs and radiation.
| Guide to Mitomycin Dose Adjustment | ||
| Nadir After Prior Dose | ||
| Leukocytes/mm3 | Platelets/mm3 | Percentage of Prior Dose to Be Given |
| ≥4,000 | ≥100,000 | 100% |
| 3,000 to 3,999 | 75,000 to 99,999 | 100% |
| 2,000 to 2,999 | 25,000 to 74,999 | 70% |
| <2,000 | <25,000 | 50% |
Dilution
Specific techniques required; see precautions.
Each 5 mg must be diluted with 10 mL SWFI. Allow to stand at room temperature until completely in solution. May be given through Y-tube or three-way stopcock of a free-flowing infusion of NS or D5W or further diluted in either of the same solutions or sodium lactate 1/6 M and given as an infusion.
Storage:
Store unopened vial at CRT. Stable after initial reconstitution at room temperature for 7 days, up to 14 days if refrigerated. When further diluted to a concentration of 20 to 40 mcg/mL, it is stable at room temperature for 3 hours in D5W, 12 hours in NS, and 24 hours in sodium lactate 1/6 M.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Sources suggest the following compatibilities:
Additive:
Manufacturer states that mitomycin (5 to 15 mg) and heparin (1,000 to 10,000 units) in 30 mL NS is stable at CRT for 48 hours. Other sources list dexamethasone (Decadron), heparin, hydrocortisone sodium succinate (Solu-Cortef).
Y-site:
Amifostine (Ethyol), bleomycin (Blenoxane), caspofungin (Cancidas), cisplatin (Platinol), cyclophosphamide (Cytoxan), doxorubicin (Adriamycin), droperidol (Inapsine), fluorouracil (5-FU), furosemide (Lasix), granisetron (Kytril), heparin, leucovorin calcium, melphalan (Alkeran), methotrexate, metoclopramide (Reglan), ondansetron (Zofran), teniposide (Vumon), thiotepa, vinblastine, vincristine.
Rate of administration
IV injection:
A single dose over 5 to 10 minutes.
Infusion:
Rate determined by amount and type of solution, typically 15 to 30 minutes.
Actions
A highly toxic antibiotic, antineoplastic agent. Cell cycle phase–nonspecific, it is most useful in G and S phases. Interferes with cell division by binding with DNA to slow production of RNA. Rapidly distributed to body tissues and ascitic fluid. Does not cross blood-brain barrier. Metabolized primarily in the liver, but some metabolism occurs in other tissues as well. Some excreted in urine.
Indications and uses
Treatment of disseminated adenocarcinoma of the stomach or pancreas. Used in combination with other drugs. Used intravesically in bladder cancer.
Unlabeled uses:
Combination chemotherapy in anal, cervical, head and neck, metastatic breast, and non–small-cell lung cancers and in malignant mesothelioma.
Contraindications
Not recommended as single-agent primary therapy. Known hypersensitivity to mitomycin, thrombocytopenia, coagulation disorders, increased bleeding from other causes, potentially serious infections, SCr greater than 1.7 mg/100 mL.
Precautions
Follow guidelines for handling cytotoxic agents. See Appendix A, p. 1331. ■ Administered by or under the direction of the physician specialist in a facility with adequate diagnostic and treatment facilities for monitoring the patient and responding to any medical emergency. ■ Use extreme caution in impaired renal function; see Contraindications. ■ Acute shortness of breath and bronchospasm have occurred within minutes to hours following administration of vinca alkaloids (e.g., vincristine) in patients who have received mitomycin previously or are receiving mitomycin simultaneously. Bronchodilators, steroids and/or oxygen may be used to treat respiratory distress. ■ Bone marrow suppression (leukopenia and thrombocytopenia) may be severe and contribute to overwhelming infections in an already compromised patient. ■ Hemolytic uremic syndrome (hemolytic anemia, thrombocytopenia, and irreversible renal failure) has occurred in patients receiving mitomycin as a single agent or in combination with other agents. It can occur at any time during treatment, but most cases have occurred with a cumulative dose greater than 60 mg. Administration of blood products may exacerbate the symptoms.
Monitor:
Monitor WBC, RBC, platelet count, PT, bleeding time, differential, and hemoglobin before, during, and 7 to 10 weeks after therapy. ■ Monitor all patients, especially those nearing a cumulative dose of 60 mg, for unexplained anemia with fragmented cells on peripheral blood smear, thrombocytopenia, and decreased renal function; see Precautions. ■ Determine absolute patency of vein; use of an IV catheter is preferred because severe cellulitis and tissue necrosis will result from extravasation. If extravasation occurs, discontinue injection and use another vein. Elevate extremity and apply cold compresses to extravasated area. Delayed erythema with or without ulceration has occurred at or distant to the injection site. May occur weeks to months after mitomycin administration, even when no obvious evidence of extravasation was observed during administration. ■ May precipitate acute respiratory distress syndrome. Oxygen can be toxic to the lungs; monitor intake carefully and use only enough to provide adequate arterial saturation. ■ Monitor fluid balance; avoid overhydration. ■ Be alert for signs of bone marrow suppression or infection. ■ Monitor for thrombocytopenia (platelet count less than 50,000/mm3). Initiate precautions to prevent excessive bleeding (e.g., inspect IV sites, skin, and mucous membranes; use extreme care during invasive procedures; test urine, emesis, stool, and secretions for occult blood). ■ Prophylactic antibiotics may be indicated pending results of C/S in a febrile neutropenic patient. ■ Prophylactic antiemetics may reduce nausea and vomiting and increase patient comfort. ■ See Precautions and Drug/Lab Interactions.
Patient education:
Nonhormonal birth control recommended. ■ Report shortness of breath and IV site burning and stinging promptly. ■ See Appendix D, p. 1333.
Maternal/child:
Avoid pregnancy; may produce teratogenic effects on the fetus. ■ Information on safety in breast-feeding or in pediatric patients not available; discontinue breast-feeding.
Elderly:
Consider diminished hepatic function; monitor for early signs of toxicity.
Drug/lab interactions
Do not administer live virus vaccines to patients receiving antineoplastic drugs. ■ May cause shortness of breath, severe bronchospasm, and acute pneumonitis with vinca alkaloids (e.g., vinblastine).
Side effects
Alopecia, anaphylaxis, anorexia, bleeding, blurring of vision, cellulitis at injection site, confusion, CHF (patient has usually received doxorubicin [Adriamycin, Doxil]), coughing, diarrhea, drowsiness, dyspnea with nonproductive cough, edema, elevated BUN or SCr, fatigue, fever, headache, hematemesis, hemolytic uremic syndrome (microangiopathic hemolytic anemia [hematocrit less than 25%], irreversible renal failure [SCr greater than 1.6 mg/dL], and thrombocytopenia [less than 100,000/mm3]), hemoptysis, hypertension, leukopenia, mouth ulcers, nausea, paresthesias, pneumonia, pruritus, pulmonary edema, purple discoloration of vein, radiographic evidence of pulmonary infiltrates, rash, renal failure, respiratory distress syndrome (acute), skin toxicity, stomatitis, syncope, thrombocytopenia, thrombophlebitis, vomiting.
Antidote
Most side effects will be treated symptomatically. Keep the physician informed. All are potentially serious and many can be life threatening. Hematopoietic depression requires cessation of therapy until recovery occurs. Discontinue drug if dyspnea, nonproductive cough, or radiographic evidence of pulmonary infiltrates is present. Discontinue drug for any symptoms of hemolytic uremic syndrome. There is no specific antidote. Supportive therapy as indicated will help sustain the patient in toxicity. Administration of whole blood products (e.g., packed RBCs, platelets, leukocytes) and/or blood modifiers (e.g., darbepoetin alfa [Aranesp], epoetin alfa [Epogen], filgrastim [Neupogen, Zarxio], pegfilgrastrim [Neulasta], sargramostim [Leukine]) may be indicated to treat bone marrow toxicity; see Precautions. If extravasation has occurred, L.A. dexamethasone injected into the indurated area with a fine hypodermic needle may be helpful; elevate extremity.
Mitoxantrone hydrochloride 
(my-toe-ZAN-trohn hy-droh-KLOR-eyed)
Novantrone
Antineoplastic (antibiotic)
pH 3 to 4.5
Usual dose
Preliminary evaluations and testing required; see Monitor.
Combination initial therapy for acute nonlymphocytic leukemia (ANLL) in adults:
Induction:
12 mg/M2/day of mitoxantrone on Days 1 through 3 and cytarabine 100 mg/M2/day as a continuous 24-hour infusion on Days 1 through 7. Should a complete remission not be achieved, repeat mitoxantrone, 12 mg/M2/day for only 2 days, and cytarabine 100 mg/M2/day for 5 days after all signs or symptoms of severe or life-threatening nonhematologic toxicity have cleared.
Consolidation:
After full hematologic recovery (usually 6 weeks after induction therapy), administer mitoxantrone 12 mg/M2/day by IV infusion on Days 1 and 2 and cytarabine 100 mg/M2/day as a continuous 24-hour infusion on Days 1 to 5. May repeat in 4 weeks. Severe myelosuppression occurred in these subsequent courses. See Monitor.
Prostate cancer:
12 to 14 mg/M2 as a short IV infusion once every 21 days. Used concurrently with steroids.
Multiple sclerosis (MS):
12 mg/M2 as an infusion over 5 to 15 minutes. Repeat every 3 months. May be given for up to 2 years or until a cumulative dose of 140 mg/M2 has been administered.
Dose adjustments
Adjust dose based on clinical response and development and severity of toxicity. ■ Clearance is reduced by impaired hepatic function. Treat patients with impaired hepatic function with caution; dose adjustment may be indicated. Specific recommendations not available; see Precautions.
Dilution
Specific techniques required; see precautions.
A single dose must be diluted with at least 50 mL of NS or D5W. May be further diluted in NS, D5W, or D5NS. Must be given through Y-tube or three-way stopcock of a free-flowing infusion of D5W or NS, or may be diluted in larger amounts of the same solutions and given as a continuous infusion.
Filters:
No data available from manufacturer.
Storage:
Store unopened vial at RT (15° to 25° C [59° to 77° F]). Do not freeze. Diluted solution should be used immediately. After penetration of the stopper on a multidose vial, the undiluted mitoxantrone may be stored at RT for 7 days or refrigerated for up to 14 days. Do not freeze.
Compatibility
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer recommends not mixing in the same infusion with other drugs until compatibility data are available and states that it may form a precipitate if mixed in the same infusion with heparin.
One source suggests the following compatibilities:
Additive:
Not recommended by manufacturer.
Cyclophosphamide (Cytoxan), cytarabine (ARA-C), etoposide (VePesid), fluorouracil (5-FU), hydrocortisone sodium succinate (Solu-Cortef), potassium chloride (KCl).
Y-site:
Allopurinol (Aloprim), amifostine (Ethyol), cladribine (Leustatin), etoposide (VePesid), etoposide phosphate (Etopophos), filgrastim (Neupogen), fludarabine (Fludara), gemcitabine (Gemzar), granisetron (Kytril), linezolid (Zyvox), melphalan (Alkeran), ondansetron (Zofran), oxaliplatin (Eloxatin), sargramostim (Leukine), teniposide (Vumon), thiotepa, vinorelbine (Navelbine).
Rate of administration
IV injection:
A single dose of properly diluted medication over at least 3 to 5 minutes. Must be given through Y-tube or three-way stopcock of a free-flowing infusion of D5W or NS.
Intermittent infusion:
A single dose over 15 to 30 minutes.
Infusion:
Sometimes a single dose is given as a continuous infusion over 24 hours. Is combined with cytarabine.
Actions
An anthracenedione, a synthetic antibiotic antineoplastic agent. Has achieved complete remissions with a single course of combination therapy. Has a cytocidal effect on proliferating and nonproliferating cells. Probably not cell-cycle specific. Inhibits DNA and RNA synthesis. Thought to reduce the number of relapses and slow down progression of MS through its ability to suppress the activity of T-cells, B-cells, and macrophages. These cells attack the myelin sheath around nerve cells, causing the symptoms of MS. Improves the presentation of brain lesions on MRI studies. Extensive distribution to tissue occurs rapidly. Partially metabolized. Exact pathways unknown. Half-life varies from 23 to 213 hours (median 75 hours). Slowly excreted in bile, urine, and feces as either unchanged drug or as inactive metabolites.
Indications and uses
Treatment of acute nonlymphocytic leukemia in adults; includes erythroid, monocytic, myelogenous, and promyelocytic acute leukemias. Given in combination with other approved drugs. ■ Treatment of bone pain in patients with advanced prostate cancer resistant to hormones. Used concurrently with steroids. ■ To reduce neurologic disability and/or the frequency of clinical relapses in patients with secondary (chronic) progressive, progressive relapsing, or worsening relapsing-remitting multiple sclerosis (i.e., patients whose neurologic status is significantly abnormal between relapses). Not indicated in the treatment of patients with primary progressive MS.
Unlabeled uses:
Treatment of acute lymphocytic leukemia (ALL), breast cancer, Hodgkin’s lymphoma, non-Hodgkin’s lymphomas, myelodysplastic syndrome, pediatric acute leukemias, pediatric sarcoma, and part of a conditioning regimen for autologous hematopoietic stem cell transplantation (HSCT).
Contraindications
Hypersensitivity to mitoxantrone or other anthracyclines. ■ Not for intrathecal use; severe injury with permanent sequelae can result.
Precautions
For IV use only. Do not administer SC, IM, intra-arterially, or intrathecally. ■ Follow guidelines for handling cytotoxic agents. See Appendix A, p. 1331. ■ Use of goggles, gloves, and protective gown recommended. Flush skin copiously with warm water should any contact occur. Irrigate eyes immediately in case of contact. Clean spills with 5.5 parts calcium hypochlorite to 13 parts by weight of water for each 1 part of mitoxantrone. ■ Usually administered by or under the direction of the physician specialist with facilities for monitoring the patient and responding to any medical emergency. ■ Will cause severe myelosuppression; use extreme caution in pre-existing drug-induced bone marrow suppression. ■ Should not be given to patients with baseline neutrophil counts of less than 1,500 cells/mm3 (except for the treatment of acute nonlymphocytic leukemia). ■ MS patients with a baseline left ventricular ejection fraction (LVEF) below the lower limit of normal or patients who have received a cumulative dose equal to or greater than 140 mg/M2 should not be treated with mitoxantrone. ■ May cause severe cardiac toxicity (e.g., acute congestive heart failure) in all patients, even if cardiac risk factors are not present. May occur early during therapy or months to years after completion. Risk increased with cumulative doses (equal to or greater than 140 mg/M2), in patients with pre-existing heart disease, and in patients previously treated with anthracyclines (see Drug/Lab Interactions), other cardiotoxic drugs, or radiation therapy encompassing the heart. ■ Use caution in impaired liver function. Clearance is decreased; see Dose Adjustments. Patients with MS who have hepatic impairment should ordinarily not be treated with mitoxantrone. ■ Use caution if renal function is impaired; has not been studied. ■ Urine and sclera may turn bluish in color. ■ Therapy with mitoxantrone increases the risk of developing secondary leukemia in patients with multiple sclerosis and in patients with cancer. Most commonly reported types are acute promyelocytic leukemia and acute myelocytic leukemia. The occurrence is more common when mitoxantrone is given in combination with other cytotoxic agents and/or radiotherapy or when doses of anthracyclines (e.g., doxorubicin [Adriamycin], idarubicin [Idamycin]) have been escalated. ■ Rapid lysis of cancer cells may cause tumor lysis syndrome. ■ See Monitor.
Monitor:
Obtain baseline CBC with differential and platelet count; repeat before each dose and if S/S of infection occur. ■ Complete a physical exam and ECG and obtain a complete history to assess for S/S of pre-existing cardiac disease. ■ In all patients, obtain an evaluation of left ventricular ejection fraction (LVEF) by echocardiogram or MUGA (multiple-gated acquisition) before therapy begins. ■ Evaluation of LVEF and assessment of cardiotoxicity by history, physical exam, and ECG should be repeated before each dose in MS patients. ■ Obtain repeat LVEF as indicated in all patients. ■ Mitoxantrone should not ordinarily be administered to MS patients who have received a cumulative dose equal to or greater than 140 mg/M2 or to patients who experience a drop in LVEF to below the lower limit of normal or a clinically significant reduction in LVEF. MS patients should have an annual quantitative evaluation of LVEF after discontinuing mitoxantrone therapy to monitor for late-occurring cardiotoxicity. ■ Monitoring of liver function is indicated before and during therapy. ■ Because of rapid lysis of cancer cells, initiate hypouricemic therapy with allopurinol or similar agents before beginning treatment. Monitor uric acid levels, maintain hydration, and alkalinize urine if necessary. ■ Observe closely and frequently for all signs of bleeding or infection. ■ Prophylactic antibiotics may be indicated pending results of C/S in a febrile neutropenic patient. ■ Determine absolute patency of vein. Severe local tissue damage may occur with extravasation. Phlebitis at the infusion site has also been reported. Should extravasation or phlebitis occur, discontinue injection and use another vein. ■ Prophylactic antiemetics may reduce nausea and vomiting and increase patient comfort. ■ Monitor for thrombocytopenia (platelet count less than 50,000/mm3). Initiate precautions to prevent excessive bleeding (e.g., inspect IV sites, skin, and mucous membranes; use extreme care during invasive procedures; test urine, emesis, stool, and secretions for occult blood). ■ Monitor for S/S of acute leukemia (secondary leukemia); may include excessive bruising, bleeding, and recurrent infections. ■ See Precautions and Drug/Lab Interactions.
Patient education:
Nonhormonal birth control recommended; see Maternal/Child. ■ Blood and cardiac tests are imperative; keep all appointments. ■ Report IV site burning or stinging promptly. ■ Urine may turn blue-green for 24 hours following administration. Bluish discoloration of sclera may also occur. ■ Medication guide available; read before beginning treatment with mitoxantrone. ■ See Appendix D, p. 1333.
Maternal/child:
Category D: avoid pregnancy. May produce teratogenic effects on the fetus. Women with MS who are biologically capable of becoming pregnant should have a pregnancy test before each dose of mitoxantrone regardless of other methods of birth control used, including birth control pills. ■ Secreted in breast milk. Discontinue breast-feeding. ■ Safety for use in pediatric patients not established.
Elderly:
Specific age-related differences have not been identified; consider age-related organ impairment (e.g., bone marrow reserve, renal, hepatic) and possibility of increased sensitivity.
Drug/lab interactions
Additive bone marrow suppression may occur with radiation therapy and/or other bone marrow–suppressing agents (e.g., azathioprine, chloramphenicol, melphalan [Alkeran]). Dose reduction may be required. ■ Risk of cardiotoxicity increased in patients previously treated with maximum cumulative doses of other anthracyclines (e.g., doxorubicin [Adriamycin], epirubicin [Ellence], idarubicin [Idamycin]) and/or radiation encompassing the heart. ■ Do not administer live virus vaccines to patients receiving antineoplastic drugs.
Side effects
Alopecia (reversible), bladder infections, menstrual disorders, mucositis, and nausea occur frequently. Other side effects include abdominal pain, acute congestive heart failure, altered electrolytes, altered liver function tests (e.g., increased ALT, AST, BUN), arrhythmias, arthralgias, bleeding, bone marrow suppression (severe with standard doses), cardiotoxicity, conjunctivitis, cough, decrease in LVEF, diarrhea, dyspnea, erythema, fever, GI bleeding, headache, hematuria, hypersensitivity reactions (e.g., dyspnea, hypotension, rash, urticaria), infections, injection site burning, jaundice, leukemia (including secondary AML), mucositis, myalgias, nail bed changes, phlebitis, renal failure, seizures, skin discoloration, stomatitis, swelling, vomiting. Interstitial pneumonitis has been reported. Anaphylaxis has been reported rarely.
Post-marketing:
Secondary acute myelogenous leukemia (AML).
Antidote
There is no specific antidote. Notify physician of all side effects. Most will be treated symptomatically. Blood and blood products, antibiotics, and other adjunctive therapies must be available. Blood modifiers (e.g., darbepoetin alfa [Aranesp], epoetin alfa [Epogen], filgrastim [Neupogen, Zarxio], pegfilgrastim [Neulasta], sargramostim [Leukine]) may be indicated to treat bone marrow toxicity. Nadir of leukocyte count occurs within 10 days. Recovery is within 21 days. For extravasation, elevate extremity and apply ice. Monitor closely and obtain surgical consult if necessary. Overdose has resulted in death. Peritoneal dialysis or hemodialysis not effective. Supportive therapy as indicated will help sustain the patient in toxicity.
Morphine sulfate 
(MOR-feen SUL-fayt)
Astramorph PF, Duramorph PF
Opioid analgesic (agonist)
Adjunct, pulmonary edema
Anesthesia adjunct
pH 2.5 to 7
Usual dose
IV injection:
Manufacturer recommends an initial dose of 2 to 10 mg/70 kg of body weight. Repeat every 3 to 4 hours as necessary. Doses may range from 2 to 20 mg based on patient requirements and response. Titrate to achieve pain relief with lowest dose. Frequent, repeated doses (e.g., up to every 5 minutes if needed) in small-dose increments (e.g., 1 to 4 mg) may be associated with fewer side effects than the administration of larger, less frequent doses.
Cancer patients suffering with severe chronic pain often require higher doses because of increased tolerance (up to 150 mg/hr has been given). Very high doses (275 to 440 mg/hr) are occasionally used for short periods of time (hours to days) for extreme exacerbations of pain in these drug-tolerant individuals. 1 to 3 mg/kg over 15 to 20 minutes will induce unconsciousness.
Acute MI:
2 to 4 mg initially. May give additional doses of 2 to 8 mg at 5- to 15-minute intervals as needed (AHA guidelines).
Infusion:
1 mg/mL (range is 0.1 to 1 mg/mL) in NS or D5W per controlled infusion device (may be patient activated). Based on a 1 mg/mL dilution, an initial loading dose may be as high as 15 mg (15 mL). The continuous background infusion to provide a level of pain relief and maintain patency of the vein may range from 1 to 2.5 mg/hr (1 to 2.5 mL). Additional doses averaging 0.5 to 1.5 mg (0.5 to 1.5 mL) may be activated by the patient at selected intervals every 3 to 60 minutes (averaging 10 to 15 minutes). Additional boluses averaging 1 to 2 mg (1 to 2 mL) may be given by health care professionals (e.g., every 30 minutes prn). In selected cancer patients all of these doses may be considerably higher.
Open heart surgery:
0.5 to 3 mg/kg as the sole anesthetic or with an anesthetic agent. Cardiovascular function not depressed if oxygen is used and adequate ventilation maintained.
Dyspnea during end-of-life care (unlabeled):
2 to 5 mg IV every 5 to 10 minutes until relief. Patient-controlled anesthesia (PCA) is recommended in the inpatient setting. Higher doses may be needed for patients taking chronic opioids.
Pediatric dose
Analgesic:
Usual range is 0.05 to 0.1 mg/kg. Administer very slowly.
Postoperative analgesia:
0.01 to 0.04 mg/kg/hr (10 to 40 mcg/kg/hr).
Selected pediatric patients with severe chronic cancer pain:
0.025 to 2.6 mg/kg/hr (average 0.04 to 0.07 mg/kg/hr).
Selected pediatric patients with severe pain during sickle cell crisis:
0.025 to 2.6 mg/kg/hr (average 0.04 to 0.07 mg/kg/hr).
Neonatal dose
Elimination is reduced in neonates, and they have an increased susceptibility to CNS side effects.
Analgesia/tetralogy (cyanotic) spells (unlabeled):
0.05 to 0.2 mg/kg/dose every 4 hours. Titrate to individual response. Another source suggests an IV injection of 0.05 to 0.1 mg/kg/dose every 4 to 8 hours or an IV infusion of 0.01 to 0.02 mg/kg/hr. Titrate to individual response.
Mechanical ventilation of neonates (unlabeled):
50 mcg/kg as an initial loading dose. Administer over 30 to 60 minutes. Follow with a continuous infusion of 10 to 30 mcg/kg/hr. Titrate to individual response.
Postoperative analgesia (unlabeled):
50 mcg/kg as an initial loading dose. Administer over 30 to 60 minutes. Follow with a continuous infusion of 15 mcg/kg/hr. Titrate to individual response.
Dose adjustments
Reduced dose and/or extended intervals may be required in impaired renal or hepatic function and in the elderly. ■ Doses appropriate for the general population may cause serious respiratory depression in vulnerable patients. ■ Increase doses as required if analgesia is inadequate, tolerance develops, or pain severity increases. The first sign of tolerance is usually a reduced duration of effect. ■ See Drug/Lab Interactions.
Dilution
IV injection:
May be given undiluted; however, further dilution with 5 mL of SWFI or NS for injection or other IV solutions to facilitate titration is appropriate. May be given through Y-tube or three-way stopcock of infusion set.
Infusion:
Each 0.1 to 1 mg is usually diluted in 1 mL NS or D5W and administered via a controlled infusion device that may be patient activated (e.g., a narcotic syringe infuser system). Available in 60-mL ampules containing 1 to 2 mg/mL for direct transfer to syringe infuser systems. (Astramorph PF and Duramorph are preservative free and expensive; can be used IV, but are the only choice for epidural or intrathecal injection; see drug literature. Duramorph is NOT for use in continuous microinfusion devices. Infumorph is NOT for IV use.) Fluid restriction or high doses may require more concentrated solutions. Concentrations above 5 mg/mL are rarely exceeded. Available in vials containing 25 mg/mL, which must be further diluted before infusion. Also available in ADD-Vantage vials for use with ADD-Vantage infusion containers. Is sometimes added to larger amounts (500 mL to 1 L) of IV solution in selected situations and infused via a large volume-controlled infusion pump (requires close titration).
Storage:
Store at CRT. Protect from light and freezing.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
One source suggests the following compatibilities:
Additive:
Alteplase (tPA, Activase), atracurium (Tracrium), dobutamine, fluconazole (Diflucan), furosemide (Lasix), ketamine (Ketalar), meropenem (Merrem IV), metoclopramide (Reglan), ondansetron (Zofran), succinylcholine, verapamil.
Y-site:
Acetaminophen (Ofirmev), acyclovir (Zovirax), allopurinol (Aloprim), amifostine (Ethyol), amikacin, aminophylline, amiodarone (Nexterone), ampicillin, ampicillin/sulbactam (Unasyn), anidulafungin (Eraxis), argatroban, atracurium (Tracrium), atropine, aztreonam (Azactam), bivalirudin (Angiomax), bumetanide, calcium chloride, caspofungin (Cancidas), cefazolin (Ancef), cefepime (Maxipime), cefotaxime (Claforan), cefotetan, cefoxitin (Mefoxin), ceftaroline (Teflaro), ceftazidime (Fortaz), ceftriaxone (Rocephin), cefuroxime (Zinacef), chloramphenicol (Chloromycetin), cisatracurium (Nimbex), cladribine (Leustatin), clindamycin (Cleocin), dexamethasone (Decadron), dexmedetomidine (Precedex), diazepam (Valium), digoxin (Lanoxin), diltiazem (Cardizem), diphenhydramine (Benadryl), dobutamine, docetaxel (Taxotere), dopamine, doripenem (Doribax), doxycycline, enalaprilat (Vasotec IV), epinephrine (Adrenalin), erythromycin (Erythrocin), esmolol (Brevibloc), etomidate (Amidate), etoposide phosphate (Etopophos), famotidine (Pepcid IV), fenoldopam (Corlopam), fentanyl, filgrastim (Neupogen), fluconazole (Diflucan), fludarabine (Fludara), foscarnet (Foscavir), furosemide (Lasix), gemcitabine (Gemzar), gentamicin, granisetron (Kytril), heparin, hetastarch in electrolytes (Hextend), hydrocortisone sodium succinate (Solu-Cortef), hydromorphone (Dilaudid), 6% hydroxyethyl starch (Voluven), insulin (regular), ketorolac (Toradol), labetalol, levofloxacin (Levaquin), lidocaine, linezolid (Zyvox), lorazepam (Ativan), magnesium sulfate, melphalan (Alkeran), meropenem (Merrem IV), methyldopate, methylprednisolone (Solu-Medrol), metoclopramide (Reglan), metoprolol (Lopressor), metronidazole (Flagyl IV), midazolam (Versed), milrinone (Primacor), nafcillin (Nallpen), nicardipine (Cardene IV), nitroglycerin IV, nitroprusside sodium, norepinephrine (Levophed), ondansetron (Zofran), oxacillin (Bactocill), oxaliplatin (Eloxatin), oxytocin (Pitocin), paclitaxel (Taxol), palonosetron (Aloxi), pancuronium, pantoprazole (Protonix IV), pemetrexed (Alimta), penicillin G potassium, phenobarbital (Luminal), piperacillin/tazobactam (Zosyn), potassium chloride (KCl), propofol (Diprivan), propranolol, ranitidine (Zantac), remifentanil (Ultiva), sodium bicarbonate, sulfamethoxazole/trimethoprim, tacrolimus (Prograf), teniposide (Vumon), thiotepa, ticarcillin/clavulanate (Timentin), tirofiban (Aggrastat), tobramycin, vancomycin, vecuronium, vinorelbine (Navelbine), warfarin (Coumadin), zidovudine (AZT, Retrovir).
Rate of administration
Frequently titrated according to symptom relief and respiratory rate. Side effects markedly increased if rate of injection too rapid. Rapid IV administration may result in chest wall rigidity.
IV injection:
15 mg or fraction thereof over 4 to 5 minutes.
Infusion:
Initial loading dose, basal rate (continuous rate of infusion), patient selfadministered dose and interval, additional boluses permitted, and total dose for 1 hour should be ordered by physician. Administer initial dose and boluses at rate for IV injection. For continuous infusion and self-administered dose and interval, note range of mL/hr under Usual Dose.
Actions
An opium-derivative, opioid analgesic that is a descending CNS depressant. Produces a wide spectrum of pharmacologic effects, including analgesia, diminished GI mobility, dysphoria, euphoria, physical dependence, respiratory depression, and somnolence. Pain relief is effected almost immediately and lasts up to 4 to 5 hours (mean is 2 hours). Morphine induces sleep and inhibits perception of pain by binding to opiate receptors, decreasing sodium permeability, and inhibiting transmission of pain impulses. Depresses many other senses or reflexes. Relieves pulmonary congestion, lowers myocardial oxygen requirements, and reduces anxiety. Metabolized in the liver and primarily excreted in the urine and feces. Crosses the blood-brain barrier, but plasma concentration of morphine is higher than CSF concentration. Crosses the placental barrier. Secreted in breast milk.
Indications and uses
Relief of moderate to severe acute and chronic pain (e.g., postoperative or cancer pain). ■ Analgesic of choice in pain associated with myocardial infarction. ■ Treatment of acute pulmonary edema associated with left ventricular failure. ■ Used before surgery to sedate, decrease anxiety, and facilitate induction of anesthesia. ■ Management of neonatal opiate withdrawal.
Unlabeled uses:
Treatment of dyspnea in end-of-life care. ■ Control of pain during mechanical ventilation in neonates. ■ Control of postoperative pain in neonates.
Contraindications
Hypersensitivity to morphine sulfate or any component of the formulation, bronchial asthma (acute or severe), and upper airway obstruction. Other sources include paralytic ileus, premature infants, or labor and delivery of premature infants. Specific formulations may have additional contraindications; see prescribing information. ■ Duramorph is NOT for use in continuous microinfusion devices.
Precautions
Schedule II opioid agonists, including hydromorphone, morphine, oxymorphone, oxycodone, fentanyl, and methadone, have the highest potential for abuse and risk of producing respiratory depression. Alcohol, CNS depressants, and other opioids potentiate the respiratory depressant effects of hydromorphone, increasing the risk of respiratory depression that might result in death; see Drug/Lab Interactions. ■ Use caution in the elderly, in patients with impaired hepatic or renal function, and in pulmonary disease. ■ May cause severe hypotension in an individual whose ability to maintain BP has been compromised by a depleted blood volume or a concurrent administration of drugs such as phenothiazines or general anesthetics; see Drug/Lab Interactions. ■ Use extreme caution in craniotomy, head injury, and increased intracranial pressure; respiratory depression and intracranial pressure may be further increased. ■ May cause sedation and pupillary changes (miosis) that may obscure the existence, extent, and course of intracranial pathology. ■ May cause apnea in asthmatic patients. ■ Symptoms of acute abdominal conditions may be masked. ■ May increase ventricular response rate in presence of supraventricular tachycardias. ■ Cough reflex is suppressed. ■ Tolerance as well as psychological and physical dependence can develop. Tolerance for the drug gradually increases, but abstinence for 1 to 2 weeks will restore effectiveness. Risk of using a narcotic antagonist in patients chronically receiving narcotic therapy should be considered. ■ A marked increase in dose may precipitate seizures. Use with caution in patients with known seizure disorders.
Monitor:
Oxygen, controlled respiratory equipment, and naloxone must always be available. ■ Observe patient frequently to continuously based on amount of dose and monitor vital signs. ■ Assess baseline pain, then assess pain with vital signs and/or more frequently if needed. Reassess after administration of morphine and adjust dose or interval as required. ■ Keep patient supine; orthostatic hypotension and fainting may occur; less likely with continuous low doses, but observe closely during ambulation. ■ Uncontrolled pain causes sleep deprivation, decreases pain threshold, and increases pain. When pain is finally controlled, expect the patient to sleep more until recovery from sleep deprivation. ■ Adhere to prescribed bowel care regimen to avoid constipation and/or impaction. Maintain adequate hydration. ■ See Drug/Lab Interactions.
Patient education:
Avoid alcohol or other CNS depressants (e.g., barbiturates, benzodiazepines [e.g., diazepam (Valium)]). ■ May cause blurred vision, dizziness, or drowsiness; use caution in tasks that require alertness. ■ Request assistance with ambulation. ■ May be habit forming.
Maternal/child:
Category C: safety for use in pregnancy or breast-feeding not established. Benefits must outweigh risks. ■ May reduce strength, duration, and frequency of uterine contractions during labor and delivery. ■ See Contraindications. ■ Pediatric patients may be more sensitive to effects, especially respiratory depressant effects. ■ May cause paradoxical excitation. ■ May cause respiratory depression in the neonate when given during labor and delivery. Have naloxone and resuscitative equipment available. ■ Infants born to mothers who have been taking morphine chronically may exhibit withdrawal symptoms.
Elderly:
See Dose Adjustments and Precautions. ■ May be more sensitive to effects (e.g., respiratory depression, constipation, urinary retention). ■ Lower doses may provide effective analgesia. ■ Consider age-related organ impairment.
Drug/lab interactions
Alcohol, other CNS depressants (e.g., narcotic analgesics, general anesthetics, antidepressants [e.g., amitriptyline (Elavil), imipramine (Tofranil), nortriptyline (Aventyl)], barbiturates, hypnotics, sedatives), H2 antagonists (e.g., cimetidine [Tagamet]), and some phenothiazines (e.g., chlorpromazine [Thorazine]) may increase CNS depression, respiratory depression, and hypotension; reduced dose of one or both agents indicated. ■ Anticholinergics (e.g., atropine) and antidiarrheals may increase risk of constipation or paralytic ileus. ■ Hypotensive effects will be increased with diuretics (e.g., furosemide [Lasix]), antihypertensive agents (especially ganglionic blockers [e.g., guanethidine (Ismelin)]), or hypotension-producing agents (e.g., antidepressants, benzodiazepines [e.g., diazepam], adrenergic blocking agents [e.g., propranolol], calcium channel blocking agents [e.g., diltiazem], calcium, nitroprusside sodium, nitroglycerin). ■ Concurrent use with rifampin (Rifadin) may decrease analgesic effects of morphine; an alternate analgesic may be required. ■ Markedly reduced doses of MAO inhibitors (e.g., selegiline [Eldepryl]) required with opiates. ■ Administration of agonist/antagonist analgesics (e.g., butorphanol [Stadol] or buprenorphine [Buprenex]) to an opiate-dependent patient receiving a pure opiate may precipitate withdrawal symptoms. ■ May potentiate anticoagulant effect of oral warfarin.
Side effects
Average dose:
Anxiety, bradycardia, confusion, constipation, decreased libido in men and women, delayed absorption of oral medications, depression of cough reflex, dizziness, drowsiness/sedation, euphoria, histamine-related reactions (e.g., local tissue irritation, pruritus, urticaria, wheals), hypersensitivity reactions, hypothermia, increased intracranial pressure, interference with thermal regulation, menstrual irregularities (including amenorrhea), nausea, neonatal apnea, oliguria, orthostatic hypotension, physical or psychological dependence, reduced male potency, respiratory depression (slight), skeletal muscle rigidity, tremors, urinary retention, vomiting. Side effects associated with histamine and constipation may be more common with morphine than with most other narcotic analgesics.
Higher doses:
CNS excitation (convulsions), respiratory depression (severe).
Overdose:
Anaphylaxis, cardiac arrest, Cheyne-Stokes respiration, circulatory collapse, coma, excitation, hypotension (severe), inverted T-wave on ECG, myocardial depression (severe), pinpoint pupils, respiratory depression or arrest, tachycardia, death.
Antidote
With increasing severity of any side effect or onset of symptoms of overdose, discontinue the drug and notify the physician. Naloxone will reverse cardiovascular, CNS, and respiratory reactions. Question the diagnosis of narcotic-induced toxicity if no response is observed after administration of 10 mg of naloxone. A patent airway, artificial ventilation, oxygen therapy, and other symptomatic treatment must be instituted promptly. Resuscitate as necessary.
Moxifloxacin hydrochloride 
(mox-ee-FLOX-ah-sin hy-droh-KLOR-eyed)
Avelox
Antibacterial (fluoroquinolone)
pH 4.1 to 4.6
Usual dose
400 mg once every 24 hours. Duration of therapy is based on diagnosis as listed in the following chart. Serum levels similar by oral or IV route. Transfer to oral therapy as soon as practical; no dose adjustment necessary. The magnitude of QT prolongation may increase with increasing serum concentrations. Do not exceed recommended dose.
| Moxifloxacin Dosing Guidelines | ||
| Infection* | Daily Dose (mg) | Duration (days) |
| Acute bacterial sinusitis | 400 mg | 10 days |
| Acute bacterial exacerbation of chronic bronchitis | 400 mg | 5 days |
| Community-acquired pneumonia | 400 mg | 7-14 days |
| Uncomplicated skin and skin structure infections | 400 mg | 7 days |
| Complicated skin and skin structure infections | 400 mg | 7-21 days |
| Complicated intra-abdominal infections† | 400 mg | 5-14 days |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


