The correct collection procedure
Before attempting venepuncture, the correct method of collection according to local policy should be ascertained and all necessary equipment assembled. There are many designated tubes available; if blood is sent to the laboratory in the wrong tube it cannot be processed, time and money are wasted and unnecessary discomfort is caused to the patient in repeating the procedure. Check unfamiliar tests with the laboratory before commencing.
If difficulties arise in collecting a blood sample from a patient it is advisable that only two attempts should be made before calling for assistance from a more experienced member of staff. Factors that can cause difficulties locating a vein include dehydration, hypotension, obesity and fragile veins.
Points for consideration
If difficulty is encountered in locating a suitable vein:
- Gently tap the chosen site and then redisinfect the site to prevent contamination.
- Ask the patient to open and close their fist several times.
- Inflate a sphygmomanometer cuff to a pressure between the patient’s systolic and diastolic blood pressure proximal to the venepuncture site.
- Hang the arm down towards the floor for a few minutes.
- In cold weather, keep clothing intact until the last moment, or apply a heat pack to the site to encourage peripheral circulation.
Haematoma formation can be prevented by applying adequate pressure to the puncture site until clotting occurs. A haemolysed sample may occur if the incorrect size of needle for the vein is used; the skin disinfectant is not allowed to dry; blood is sampled from an IV cannula or central venous catheter; the blood sample tube is underfilled; too much force is used drawing back or to mix the sample; overnight storage such as an electrolyte sample (WHO 2010).
Blood samples from dialysis access needles or lines must be free of saline and anticoagulants (for example, heparin), be free of clotted material, be blood taken directly from the patient and not the machine and must not be recirculated blood. Blood for any clotting tests should be taken predialysis from another site such as the dorsal of the hand or antecubital fossa, as any heparin contamination will falsify the result. Haemodialysis samples should all have ‘pre-, mid- or post-dialysis sample’ clearly marked on the request form to avoid confusion.
Key points
- Check the tourniquet is clean prior to use as they are a potential meticillin resistant Staphylococcus aureus (MRSA) source.
- Always check that the correct collection tube and request form are to hand.
- Know whether the sample has to be delivered immediately to the laboratory, or can be stored at room temperature, in a fridge or on ice.
- Check whether the patient should be fasting.
- Use minimum pressure with a tourniquet to avoid haemolysis, and also to minimise bruising.
- Ensure the sample is correctly labelled.
- Use biohazard labels for known contaminated samples (as per local protocol). However, all samples should be treated as potentially hazardous.
Biochemical, Blood Tests
Normal values are listed in Table 7.1. These may vary locally and may be expressed in alternative units of measurement. Paediatric normal values should always be checked (see Chapter 12).
Table 7.1 Analysis of a normal blood sample.
| Urea (blood urea nitrogen) | Adult 2.5–6.4 mmol/l |
| Child 1.1–6.4 mmol/l | |
| Creatinine | Adult 70–120 µmol/l |
| Child (increasing with age) 27–88 µmol/l | |
| Sodium | 135–147 mmol/l |
| Potassium | 3.5–5 mmol/l |
| Calcium | 2.2–2.65 mmol/l |
| Phosphate | 0.8–1.5 mmol/l |
| Bicarbonate | 22–30 mmol/l |
| Cholesterol | 3.5–5.7 mmol/l |
| Total protein | 60–80 g/l |
| Albumin | 35–55 g/l |
| Glucose (fasting) | 3.6–5.8 mmol/l |
Monitoring the blood biochemistry of patients with CKD is central to their diagnosis and ongoing care, as this reflects the kidneys’ function in excreting the waste products of metabolism. Serum or heparinised plasma samples are suitable for most biochemistry investigations.
Blood to be separated for serum samples is collected in a plain clotting tube (no additives) or in a tube containing beads treated with a clotting activator.
Blood to be separated for plasma samples is collected in a tube containing lithium heparin or beads treated with lithium heparin. The beads form a layer between the blood clot and serum or plasma after centrifugation, which allows serum to be withdrawn by pipette for the appropriate analysis. Some tubes contain a gel for the same purpose to act as a barrier between cells and plasma or serum.
Renal profile
The following tests (urea, creatinine, sodium, potassium, corrected calcium, phosphate, bicarbonate and albumin) are often requested together. The result is generated from one 5 ml blood sample in a plain or lithium heparin tube. Some centres may include other tests under this profile.
Urea
Urea (normal range 2.5–6.4 mmol/l) is one of the principal end-products of protein metabolism. Urea is formed in the liver, carried by the blood and excreted by the kidneys in the urine. Raised blood urea indicates failure of the kidneys and usually increases in tandem with serum creatinine levels in CKD and ESKD. However, serum urea levels may remain within normal limits whilst serum creatinine levels increase.
Urea can rise dramatically in previously healthy individuals who experience overwhelming infection or major crush injuries and are admitted to hospital with acute kidney injury (AKI). A slight rise in urea may be seen if a very high protein diet is consumed, and in low-protein diets a lower level of blood urea may be observed. Certain drugs, such as corticosteroids and tetracycline, can cause a sudden rise in blood urea, especially if the patient already has CKD.
Nonrenal causes of increased urea levels
- High-protein diet
- Chronic malnutrition – increased protein metabolism.
- Gastrointestinal bleeds – increased protein absorption.
- Dehydration – increased urea reabsorption.
A normal or low urea level is not necessarily indicative of adequate dialysis if a patient is malnourished with a low protein intake.
Creatinine
Creatinine (normal range 70–120 µmol/l) is produced by the breakdown of creatine phosphate in muscle by catabolism and is excreted by the kidney. Creatinine levels may not show a significant increase until there is a 50% loss of kidney function. Therefore, serum creatinine is not a sensitive test for early kidney disease. Estimated glomerular filtration rate (eGFR) is now recommended (see Chapter 6). However, elevated creatinine levels can reliably be used as a specific indicator of kidney dysfunction as it is fairly constant from day-to-day and rises steadily with progressive renal impairment. A higher level of serum creatinine may be expected with large muscle mass, in males, and in those of African-Caribbean ethnicity. A lower level may be seen in those with a low muscle mass (for example, the elderly or those with an amputation), those who are malnourished and/or with the use of some drugs such as trimethoprim and amiloride.
In advanced kidney disease, creatinine levels may eventually rise to a level where it is considered expedient to commence dialysis. This may be in the region of 500–1000 µmo/l but varies with the individual, the symptoms experienced, and the policy of the nephrology service. Those with diabetes are usually commenced on dialysis earlier, sometimes with serum creatinine levels of 350–500 µmol/l. Creatinine levels can be plotted on a log graph at regular intervals over a period of time, and used as a predictor of the time when RRT is likely to be necessary (http://renux.dmed.ed.ac.uk/edren/Handbookbits/HDBKgfrest.html, accessed 20 May 2013).
The NICE CKD guidance (National Institute for Health and Clinical Excellence 2008) recommends the use of eGFR, alongside serum creatinine to measure kidney function. Further discussion about the limitations of reporting serum creatinine alone and ways in which eGFR can be calculated can be found later in this chapter and also in Chapter 6. The NICE guidance (2008) recommends that patients should not eat cooked meat for at least 12 hours prior to a serum creatinine test.
Sodium
Sodium, Na+ (normal range 135–147 mmol/l) is the principal electrolyte of the extracellular fluid of the blood, maintaining osmotic pressure, and is involved in acid–base balance and the transmission of nerve impulses. Sodium is taken into the body with the diet and is conserved or excreted by the kidneys. Hyponatraemia (<135 mmol/l ) can be an indication of excess body fluid, and is also often present in burns, diarrhoea, vomiting, nephritis, neoplasms and diabetic acidosis.
Hypernatraemia (>148 mmol/l) can be an indication of dehydration and insufficient water intake, multiple myeloma, diabetes insipidus, metabolic acidosis or excessive intravenous isotonic fluids in advanced kidney disease. Patients may be proportionally hypernatraemic or hyponatraemic without an altered fluid state.
Potassium
Potassium, K+ (normal range 3.5–5 mmol/l) is the principal electrolyte of the intracellular fluid, with only low concentrations (2%) circulating in the extracellular fluid. Potassium is provided by the diet and excreted mainly by the kidneys, where regulation of the body potassium content occurs – a small amount is also lost in the faeces. Potassium is necessary to maintain nerve conduction and plays a major role in control of cardiac output. Potassium levels usually remain normal if a urine output in excess of 1500 ml day can be maintained. Hypokalaemia (<3.5 mmol/l) may be found in cases of diarrhoea, vomiting, renal tubular acidosis, diuretic usage, intravenous fluid administration without added potassium, and when excess insulin causes an increase in the cellular uptake of potassium. Hypokalaemia can cause cardiac arrhythmias.
Hyperkalaemia (>5.5 mmol/l but sometimes defined as >6.0 mmol/l for those on haemodialysis) may be seen in advanced kidney disease, burns, insulin deficiency, post-traumatic conditions (including surgery), disseminated intravascular coagulation or when potassium-sparing diuretics are used with Slow-K, and in any condition where cell damage has occurred causing leakage of intracellular potassium into the extracellular fluid. In CKD a potassium level >6.5 mmol/l may be a medical emergency requiring immediate instigation of dialysis or other treatments. If left untreated, it may result in cardiac arrest caused by the arrhythmic effect of potassium buildup. Blood samples for accurate potassium analysis should be delivered swiftly to the laboratory or, if this is impossible, separated and stored, to prevent leaching of intracellular potassium into the serum, which results in a falsely raised level.
Calcium
Calcium, Ca2+ (normal range total Ca2+ 2.2–2.6 mmol/l) is provided by the diet, and is excreted by the kidneys. Most body calcium is found in the skeleton but a small proportion is circulated in the blood. About 50% of serum calcium is protein-bound and 50% is ionized. Ionised serum calcium is responsible for muscle contraction, cardiac function and blood clotting. Corrected calcium estimates the total concentration of calcium as if the albumin concentration was normal, i.e estimates the free calcium, and is calculated as follows:
Corrected calcium (mmol/l ) = Ca2+ + ( 40 – albumin (g/l) ) × 0.02. It is the corrected calcium value that is reported by the laboratory.
In the healthy individual, calcium homeostasis is controlled by parathyroid hormone, vitamin D and the hormone calcitonin. Hypocalcaemia is found in CKD where phosphate retention is present (see Chapter 13). Chronic hypocalcaemia causes an excess of parathyroid hormone to be excreted into the blood stream which in turn releases calcium from the bone, resulting in the mineral bone disease often seen with vitamin D deficiency in CKD. In nephrotic syndrome low levels of calcium will be found due to albumin leaking into the urine, taking bound calcium with it. In the patient with nephrotic syndrome, the ratio of protein-bound and ionised calcium will remain the same. The acidosis of CKD is an added cause of loss of bone calcium. Hypercalcaemia may be an indication of hyperparathyroidism, sarcoidosis or malignancy. High levels of calcium can cause kidney stones and renal tubular disease.
Phosphate
Phosphorus, is found in the diet. Phosphate, PO43– (normal range 0.8–1.5 mmol/l) is mainly combined with calcium in the skeletal bone. It is controlled, with calcium, by parathyroid hormone and apart from its skeletal function has a part in the metabolism of glucose and lipids. Phosphates are excreted by the kidney. When phosphate is increased, calcium is lowered and vice versa.
Hypophosphataemia may be found in the patient with renal tubular disease who loses phosphate, possibly leading to osteomalacia. Hyperphosphataemia will often be found in conjunction with hypocalcaemia.
Parathyroid hormone
Normal values of parathyroid hormone (PTH) vary according to the local assay method being used. Parathyroid hormone is a hormone produced in the parathyroid gland and is involved with the regulation of extracellular calcium. This test is useful in establishing whether hypercalcaemia is due to an overactive parathyroid. Increased parathyroid hormone is common in stage 3 CKD, vitamin D deficiency and osteomalacia. Hyperparathyroidism has been implicated in reduced response to anaemia treatments and possibly due to the effect on endogenous eryhtropoeitin synthesis, bone marrow progenitors, red cell survival or as a result of indirectly causing bone marrow fibrosis (National Institute for Health and Clinical Excellence 2011). Blood for parathyroid hormone analysis should be delivered immediately to the laboratory for analysis or, if this is impossible, kept on ice for a maximum of 30 mins.
Other biochemical blood tests
Uric acid
Uric acid (normal range female 200–350 µmol/l male 260–500 µmol/l) is an end-product of purine metabolism and is excreted mainly by the kidney, but in part by the bowel. In gout, excess uric acid crystallises in joints. In CKD there is an impaired ability to excrete uric acid and a high serum level may be found in association with raised urea and creatinine. Increased serum uric acid is also found in pre-eclampsia of pregnancy, leukaemia, multiple myeloma, various cancers and in acute shock.
Bicarbonate
The normal range of HCO3– is 22–30 mmol/l. Low plasma HCO3– indicates metabolic acidosis caused by CKD with an inability to excrete hydrogen ions adequately.
Glucose
The normal range of fasting serum glucose is 3.6–5.8 mmol/l. Blood glucose levels are maintained by the liver, which absorbs and stores glucose as glycogen and releases it into the circulation in response to the demands of the body. Glucose is regulated by insulin which is synthesised by the beta cells in the islets of Langerhans in the pancreas.
Glycosylated haemoglobin
Glycosylated haemoglobin, more commonly known as HbA1c indicates the amount of glucose carried by red blood cells in the body and is a more accuarte measure of long-term glucose control in people with diabetes. The target HbA1c is <48 mmol/mol (6.5%), or a higher target if the person is at risk of hypoglycaemia (<58 mmol/mol (7.5%)). HbA1c provides an average blood glucose level over a 8–12 week period (the average life of a red blood cell), however it may underestimate blood glucose control in advanced kidney disease or conditions where the red blood cell lifespan is reduced, for example sickle-cell disease, with the use of ESAs, or produce a higher results in uraemia.
Serum protein electropheresis
Serum protein electrophoresis uses an electrical field to separate out the proteins in the serum and helps to diagnose certain diseases such as multiple myeloma.
Lipids
Cardiovascular disease is a major cause of morbidity and mortality in patients with kidney disease (Jun et al. 2011) and one of the main risk factors for this is dyslipidaemia. It is recommended by the National Institute for Health and Clinical Excellence (2010) to perform both total cholesterol and LDL cholesterol to best calculate cardiovascular risk. Hyperlipidaemia is often found in those with CKD, especially those with nephrotic syndrome and in transplant recipients. The patient should be advised to fast 10–12 h prior to the test. The normal targets are: total cholesterol ≤ 5 mmol/l and for those at high risk of cardio-vascular disease (CVD) ≤ 4 mmol/l ; LDL cholesterol ≤ 3 mmol/l (those at high risk ≤ 2 mmol/l); HDL cholesterol ≥ 1 mmol/l and triglycerides < 2.0 mmol/l.
Liver function tests (LFTs)
This is a collection of tests that include serum albumin levels, total plasma protein, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl-transpeptidase (GGT), bilirubin levels and clotting to assess liver function.
Albumin
The normal range of albumin is 35–55 g/l. Albumin levels can be elevated in patients with dehydration. Low albumin levels are common in CKD due to protein loss through peritoneal dialysate, poor dietary intake and nephrotic syndrome. Other causes of low albumin levels are decreased absorption in liver disease and increased breakdown in malignancy.
Plasma total protein
The normal range of plasma total proteins is 60–80 g/l. Hypoproteinaemia associated with low albumin (normal value 35–55 g/l ) levels may be found in the many conditions associated with nephrotic syndrome where protein leakage occurs from the kidney into the urine. Also, decreased total protein in conjunction with low albumin may be found in liver disease, burns and haemorrhage. Hyperproteinaemia (increase of total plasma protein) with a normal albumin/globulin ratio may occur in dehydration. If total protein increases with a falling albumin/globulin ratio (i.e. a raised globulin), this may indicate autoimmune disease such as systemic lupus erythematosus, shock, chronic infection or myeloma.
Alkaline phosphatase is a protein involved in cellular metabolism which is mainly produced in the bones, and bile ducts of the liver though some is produced in the kidneys and intestines. An elevated ALP is seen in mineral bone disease, liver damage and pregnancy.
Alanine aminotransferase and AST are enzymes found in high concentrations in the heart, liver and skeletal muscle. Increased levels most commonly indicate liver diease, for example hepatitis.
Gamma glutamyl-transpeptidase is an hepatic enzyme, elevated levels may be seen with liver disease, heart failure and with alcohol use and with some medications.
Bilirubin (normal range 3–17 µmol/l ) is a bile pigment produced by the breakdown of haem and reduction of biliverdin. Unconjugated bilirubin is insoluble in plasma unless bound to protein, mainly albumin. Salicylates, sulphonamides, nonesterified fatty acids and reduced pH levels result in decreased protein-binding of unconjugated bilirubin. Normally, 95% of the circulating bilirubin is unconjugated. Raised levels occur with increased production, for example in haemolysis or in hepatobiliary disease or obstruction. Jaundice or icterus describes the yellow staining of the tissues due to an excess of bilirubin – unconjugated or conjugated. Jaundice becomes clinically detectable at levels > 40 µmol/l.
Serology
Serological tests are frequently required as renal impairment is often a manifestation of a systemic disease. Many kidney disorders arise from immune dysfunction and serology will often, therefore, provide an exact diagnosis. A positive antineutrophil cytoplasmic antibody (ANCA) test is found in diseases such as systemic and renal vasculitis. Antiglomerular basement membrane (anti-GBM) is detected in Goodpasture’s syndrome. The presence of other immunoglobulins may indicate other autoimmune diseases e.g. antinuclear antibodies may be found in Systemic Lupus Erythematosus (SLE).
Complement
The most common complement studies performed are for C3 and C4. These levels rise during an acute inflammatory state. However, there are many other specialised complement studies that can be undertaken in order to diagnose a particular disease process.
Creatine kinase
An enzyme present in heart and skeletal muscle which is elevated in myocardial infaction and rhabdomyolysis.
Immunoglobulins
Immunoglobulins (five groups – IgD, IgE, IgG, IgM and IgA) are proteins present in both circulating blood and in the cells which function as antibodies defending the body against infection. Elevated levels may be seen in autoimmune disease, cancers and allergic reactions, for example IgA nephropathy and multiple myeloma. Cryoglobulins are abnormal immunoglobulins that are found in diseases such as multiple myeloma, autoimmune disease, such as systemic lupus nephritis, and infections such as hepatitis.
Viral serolgy
Screening for hepatitis B, C and HIV is required for at risk groups and those requiring RRT. HIV and Hepatitis B and C can also be a primary cause of kidney disease.
Haematology
Haematological tests give information about anaemia, haematological malignancies and clotting disorders. Infections, inflammatory disease and other conditions can be indicated by changes in total and differential white cell counts. Normal values are shown in Table 7.2 (there may be slight local variations, especially in the paediatric normal range).
Table 7.2 Normal values – haematological tests.
| Haemoglobin | Male 13.5–18 g d/l |
| Female 11.5–16.5 g d/l | |
| Haematocrit | Male 40–55% |
| Female 35–45% | |
| Platelets | 150–350 × 109/l |
| Leukocytes (white bloodcells) | 4.5–10 g/l |
Full blood count (FBC)
This test includes haemoglobin, heamatocrit, red blood cell count, white blood-cell count and differential, platelet count and blood film, which often provide evidence of renal anaemia in CKD. It may be appropriate to investigate further those values falling outside normal parameters.
Haemoglobin
Haemoglobin (Hb) should be checked to ensure that anaemia is not present. Haemoglobin varies with age, gender and ethnicity. The level at which it is considered that an ESA should be initiated varies from one centre to another, but current NICE guidance (2011) recommends an aspirational haemoglobin range of 10–12 g/dl for adults, young people and children (2 years and older) and investigations for anaemia should be undertaken if Hb < 11 g/dl (National Institute for Health and Clinical Excellence 2011). Normocytic erythrocytes are typical in the patient with renal anaemia. However, in the case of iron-deficiency anaemia, microcytic and hypochromic red blood cells will be seen.
Haematocrit
Haematocrit (HCT) is the percentage of red blood cells in the whole blood volume, which will be low in the patient with renal anaemia, running in parallel with the low level of haemoglobin. When the patient responds to the effect of erythropoeitin, a rise in haematocrit will be seen in conjunction with a rise in haemoglobin and red blood cells.
Red cell count
Red cell count (RCC) and mean corpuscular volume (MCV) reflect the size, and mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) reflect the haemoglobin concentration, of individual cells (normal ranges: MCV 76-96 fL; MCH 27–32 pg/cell (1.68 – 1.92 fmol/cell); and MCHC, 30-36g/dl). These red blood cell indices are useful in the diagnosis of types of anaemia.
Anaemias are classified on the basis of cell size (MCV) as microcytic, normocytic or macrocytic, and on the basis of the amount of haemoglobin (MCH) as hypochromic, normochromic or hyperchromic. They can therefore be classified as follows:
- Normocytic/normochromic anaemia – from acute blood loss, prosthetic heart valves, sepsis, tumour, or aplastic anaemia.
- Microcytic/hypochromic anaemia – from chronic blood loss, iron deficiency, lead poisoning, or thalassaemia.
- Microcytic/normochromic anaemia – erythropoietin deficiency in CKD.
- Macrocytic/normochromic anaemia – from chemotherapy, folate deficiency, or vitamin B-12 deficiency.
White blood cell count and differential
White blood cells are the cells in the body that fight against infections and allergies. There are five types of white blood cells, which can be split into two groups – granulocytes and agranulocytes. The granulocytes include neutrophils, eosinophils, and basophils and have granules in their cell cytoplasm, they also have a multilobed nucleus. Agranuloctye white blood cells, lymphocytes and monocytes, do not have granules and have nonlobular nuclei. They are sometimes referred to as mononuclear leukocytes.
The normal range for total white blood cell is 4–11 × 109/l. A low white cell count is referred to as leukopenia and a high white cell count as leukocytosis. Leukocytosis is usually due to an increase in one of the five types of white blood cells and is given the name of the cell that shows the primary increase (i.e., neutrophilia, eosinophilia, basophilia, lymphocytosis and monocytosis). Leucocytosis may be indicative of an infection, inflammation or a haemotolgic malignancy and leukopenia may be due to bone suppression or replacement, hypersplenism or deficiencies of cobalamin or folate.
Differential (or relative value)
This is a count of the five different types of white blood cells and is often expressed as a percentage of the total white cell count (rather than their absolute value).
- Neutrophils: 50–70% relative value. An increase may indicate infection and is called neutrophilia. A decrease is called neutropenia and may be due to chemotherapy.
- Eosinophils: 1–3% relative value. An increase may indicate infections or allergies.
- Basophils: 0.4%–1% relative value. Basophilia is an uncommon cause of leukocytosis but can be caused by infections or inflammatory conditions such as inflammatory bowel disease, chronic airway inflammation.
- Lymphocytes: 25–35% relative value. Absolute lymphocytosis may be caused by acute infections (cytomegalovirus infection, Epstein–Barr virus infection, pertussis, hepatitis, toxoplasmosis); or chronic infections (tuberculosis, brucellosis); or lymphoid malignancies (chronic lymphocytic leukaemia). Relative lymphocytosis is seen in the acute phase of several viral illnesses, in connective tissue diseases, thyrotoxicosis, Addison’s disease and splenomegaly with splenic sequestration.
- Moncocytes: 4–6% relative value. Monocytosis may be due to either chronic infection, chronic inflammatory disorders such as Crohn’s Disease or carcinoma. However a transient monocytosis can be seen with the resolution of an infection. A monocytosis may also be seen in the myelodysplastic conditions.
Coagulation
In circulating blood a series of factors are present that provide the means for clot formation as appropriate when damage to a vessel occurs. Prior to many kidney procedures, such as kidney biopsy, it is standard practice to ascertain that the patient has normal clotting function to avoid the ris k of haemorrhage. Those with uraemia are more prone to bleeding as urea affects the clotting cascade.
Included in this group of tests are platelets (normally, 150–400 × 109/l included in the FBC). Platelets adhere to each other and initiate the clotting cascade when damaged endothelium is encountered. Platelet deficiency (thrombocytopenia) is a common cause of prolonged bleeding.
Other coagulation studies likely to be encountered in kidney investigations include the bleeding time (normal <10 min); partial thromboplastin time (PTT); activated partial thromboplastin time (APTT); fibrinogen and international normalised ratio (INR). Most methods in current use require a very precise amount of blood in coagulation tests; the blood sample should exactly reach the marked line. Blood for coagulation studies during or immediately post-haemodialysis or from heparinised lines (for example, temporary or permanent dialysis catheters) should not be taken from a central line as the result will be inaccurate – it is recommended to use a vein instead.
Further investigations for renal anaemia
Anaemia is a major complication of CKD and a contributory factor to cardiovascular disease in patients needing dialysis. The major cause is the lack of production of the hormone erythropoeitin which is produced by the kidney. There are clinical benefits associated with correcting renal anaemia, including improved cardiac function, quality of life of dialysis patients and reducing the decline in kidney function in the early stages of CKD (Locatelli et al. 2007). Anaemia treatments are discussed further in Chapter 6.
Symptoms of anaemia
These include lethargy, dyspnoea, headache, dizziness, palpitations and pallor, decline in exercise tolerance and sexual function and cognitive function.
Prior to the commencement of treatment, some basic investigations must be completed in order to correct any deficiencies which may prevent an adequate response to this very expensive therapy. It is also important to exclude or treat (if possible) underlying causes such as:
- iron deficiency
- blood loss;
- infection or inflammatory disease;
- hyperparathyroidism;
- aluminium toxicity;
- vitamin B12 and folate deficiency;
- haemolysis;
- haemoglobinopathies.
Other anaemia investigations
Having ascertained that the patient has renal anaemia, the next step is to carry out certain investigations to check that there is no condition present which may prevent or reduce the effect of ESAs. These tests should be repeated if diminished or nonresponse to ESAs occurs at a later date.
Haematinics
In order to maintain the haem component of the healthy red blood cell, an adequate amount of available and stored iron must be present. There are several tests which can be carried out to determine this very important factor – the main cause of nonresponse to ESAs has been found to be low available iron. Iron-deficiency anaemia is either absolute or functional.
Ferritin
The normal range in health is 30–200 μg/l. Iron deficiency anaemia in CKD is indicated if less than 100 μg/l and should be maintained greater than 200 μg/l. Ferritin is the main form of stored iron found in all tissues, but especially in the liver, spleen and bone marrow. Ferritin found in the serum relates to the amount of stored iron, but is not necessarily an accurate assessment of available iron. Unless ferritin levels are at least 100 μg/l before commencing treatment with ESAs therapy, the response will be short lived. Ferritin levels should be kept in excess of this by infusing intravenous iron to allow adequate erythropoiesis, as oral iron supplementation is inadequate. A false high level can be seen in infection and inflammatory states, indicated by an elevated CRP (see the following section on ‘Other Tests’).
Transferrin saturation rate
Iron is transported by the specific plasma protein transferrin (or siderophilin). A useful test of available iron for red cell production is the transferrin saturation rate. Transferrin saturation indicates how much iron is circulating in the plasma relative to total iron-binding capacity. Less than 20% is indicative of iron deficiency anaemia, however it is not a reliable test and requires seqential readings to determine an average.
Serum iron
Serum iron (standard reference range varies between laboratories) in those with CKD is of no great significance (except in iron overload) but is necessary for calculating transferrin saturation rate.
Red-cell folate
Folic acid is a water-soluble vitamin in the B-complex group that is absorbed from the duodenum and jejunum. Folic acid works along with vitamin B12 and vitamin C to help the body digest and utilise proteins and to synthesise new proteins when they are needed. It is necessary for the production of red blood cells and for the normal DNA synthesis and affects erythrocyte precursors. Folic acid also helps with tissue growth and cell function. Low levels can cause macrocytic anaemia. Stores of this vitamin last only a few months.
Vitamin B12
Vitamin B12 (normal range 150–1000 ng/l) is a water-soluble vitamin that is part of the vitamin B complex. It is absorbed in the ileum. The uptake is dependent on the production of acid and intrinsic factor in the stomach, adequate oral intake and production of transcobalamin (transport protein). Vitamin B12, like the other B vitamins, is important for metabolism, and helps in the formation of red blood cells and in the maintenance of the central nervous system. Although the body stores can last several years without oral intake, low levels (e.g. in pernicious anaemia) can cause a macrocytic anaemia.
As folic acid and vitamin B12 are water soluble they are both lost during haemodialysis which can lead to dialysis-induced deficiency.
Other tests
C-Reactive Protein (CRP)
C-reactive protein (CRP) is a globulin that is synthesised by the liver and is present in small amounts in a normal individual. An elevated CRP indicative of infection, inflammation or malignancy. The most important role of CRP is its interaction with the complement system, which is one of the body’s immunologic defense mechanisms. It is normally present in the plasma at a concentration of less than 5 mg/l.
C-reactive protein increases in virtually all conditions associated with tissue damage and may double its concentration every 6 h. It is better than ESR for monitoring fast changes as it does not depend on fibrinogen or immunoglobulin levels, and is not affected by red blood cell numbers and shape.
Haptoglobins
Haptoglobin is an acute-phase protein, rising in concentration during acute inflammation. Classically, a low haptoglobin concentration is indicative of intravascular haemolysis. It may also occur in extravascular haemolysis – some free haemoglobin leaks from the phagocytic cells of the spleen, chronic liver disease, metastatic malignancy and sepsis.
Coombs’ test
The Coombs’ test is used in the investigation of haemolytic anaemia. A positive Coombs’ test is found in cases of autoimmune haemolysis due to the presence of immunoglobulin G (IgG), complement or both, on the surface of the red cells. A positive result may be found in a haemolytic transfusion reaction or autoimmune haemolysis, including drug-induced haemolysis.
Lactate dehydrogenase (LDH)
This is an enzyme that is found throughout the body in the tissues with low levels found in the circulating blood. Elevated levels occur when there is damage to the tissue as they enzyme is released from body tissue in to the bloodstream. It is used to assess acute and chronic liver damage, monitor progressive diseases, for example liver, kidney, malignancy and to diagnose haemolytic anaemia.
Occult blood
A faecal occult blood test (FOBT) is a noninvasive test that detects the presence of hidden (occult) blood in the stool. Such blood may arise from anywhere along the digestive tract. Hidden blood in stool is often the first, and in many cases the only, warning sign that a person has colorectal disease, including colon cancer. A positive test result requires further investigation of the gastrointestinal tract, usually with a colonoscopy in the first instance.
Reticulocytes
Reticulocytes (normal range in men 0.5–1.5%, in women 0.5–2.5%) are immature red blood cells that have been newly released from the bone marrow, and can be recognised as such for about 48 h before reaching a mature state. Patients with renal anaemia have a depressed reticulocyte count before ESA therapy and a rise should be seen when stimulation of erythrocyte production occurs as a response to ESAs. If no response occurs, further investigation should be considered.
The percentage of hypochromic red blood cells will assess how much iron is being incorporated into the red blood cell. This level should be <6%, greater levels indicate iron-deficiency anaemia.
Poor response to ESA therapy
Low or nonresponse to ESA therapy other than reduced haematinic levels requires investigation to assess adherence to treatment regimen and to exclude other causes such as iron deficiency anaemia, infection or inflammation, nonadherence to anaemia treatment, occult malignancy, bone marrow disorders, hyperparathyroidism, inadequate dialysis, ACEI and immunosuppressive drugs, aluminium toxicity, carnitine deficiency, chloramine toxicity in haemodialysis patients only and chronic blood loss (for example, haemorrhoids, menorrhagia, gastrointestinal bleeding), pure red cell aplasia (PRCA).
Urine investigations
Urinalysis plays an important part in the assessment of kidney disease, as kidney damage may allow increased concentrations of various chemicals through to the urine together with other signs of disease such as haematuria or proteinuria. The quantity of urine passed during the day together with its specific gravity also gives an indication of kidney function. Table 7.3 lists the normal volumes of urine passed per day.
Table 7.3 Normal volumes of urine
| Healthy adult | 1–1.5 l/day |
| Newborn baby | 50–300 ml/day |
| Infant | 350–550 ml/day |
| Child | 500–1000 ml/day |
| Adolescent | 700–1400 ml/day |
Urine is composed of about 95% water and 5% solids, mainly urea and sodium chloride, it is slightly acidic (pH 6.0) and it has a specific gravity of 1.010–1.030 (specific gravity of water = 1.000).
Urinalysis
Measurement of specific gravity can be unreliable in the presence of water and electrolyte imbalance, low-protein diets, chronic liver disease and pregnancy.
Appearance
Urine can vary in colour from pale straw to dark amber:
- Pale urine is dilute because of:
- heavy fluid intake;
- polyuria due to kidney disease where the tubules fail to reabsorb water;
- diabetes insipidus or diabetes mellitus.
- heavy fluid intake;
- Dark urine may indicate:
- concentration due to fluid depletion
- presence of bile.
- concentration due to fluid depletion
- Haematuria can vary in appearance from ‘smoky’ to ‘tea’ to red, either bright or dark.
- Coloured urine can be caused by blackberries, beetroot in the diet and other vegetable food dyes, porphyria and some medications, for example, orange-coloured urine is caused by rifampicin or senna.
- Frothy urine indicates heavy proteinuria, however may occur with an old urine sample
- Smoky urine may indicate the presence of bleeding from the kidney.
- Deposits or cloudy/turbid urine, which occurs when the urine sample is left to stand, may be crystals of phosphate, oxalate or urates, or due to pus in the presence of infection.
- Pink to black/brown (coca cola) urine may indicate the presence of myoglobin
Dipstick tests
Dipstick tests can be carried out in the clinic or ward situation as well as in the laboratory. Dipsticks are available that accurately show the presence of a variety of substances which may occur in the urine (for example, protein, glucose, ketones, blood, leuckocytes and nitrites) as well as giving the pH of the urine sample. The stick should be briefly dipped into a fresh sample of urine and read after 1 min or according to the manufacturer’s instructions. The results are then compared with those supplied on the instruction sheet.
Caution
These kits are very reliable providing that the container is always kept dry and capped between use, the strips are only briefly dipped into the urine sample and the expiry date is not exceeded.
Osmolality
Osmolality (normal 500–800 mOsmol/kg) measurement indicates the kidney’s ability in concentration and dilution and is considered more reliable than measuring the specific gravity and requires collection of an early morning urine sample.
Glucose
The presence of glucose may indicate diabetes mellitus, proximal tubular dysfunction, Fanconi’s syndrome, glomerulonephritis or nephrotic syndrome.
Blood
The presence of blood in the urine is either microscopic (only visible under microscope) or macroscopic (visible to the naked eye). Microscopic haematuria is often an incidental finding and requires further investigation to assess for kidney disease or maligancy if > +1 on two or three separate occasions and significant if > 2 per high powered field (hpf). It is also important to establish the cause is not related to a urinary tract infection, rule out other causes such as menstruation, sexual intercourse, trauma e.g. urinary catheter, too much exercise or an old sample (lysis of red blood cells).
Protein
Although protein is a normal urinary constituent, its level should be no more than a trace (i.e. < 20 mg /24 h, mainly albumin). Proteinuria may however be present at a level of 150 mg in 24 h before a dipstick test shows a positive reading. Causes of transient proteinuria include acute metabolic crisis, intercurrent illness, urinary tract infection and contamination. Persistent proteinuria is a common sign of many forms of kidney disease. In nephrotic syndrome, proteinuria may be as high as 350 mg/ 24 h. It is important to rule out a urinary tract infection or orthostatic hypotension.
The measurement of microalbuminuria/proteinuria can be used for the early detection of kidney disease and if not treated can lead to deterioration of kidney function. It is also a strong predictor of cardiovascular disease and death. NICE CKD guidance (National Institute for Health and Clinical Excellence 2008) recommends the use of albumin:creatinine ratio (ACR). An ACR measures the concentration of albumin and creatinine and has a greater sensitivity in detecting low levels of proteinuria and should be used for diabetes (normal range: ACR < 2.5 mg/mmol in men and <3.5 mg/mmol in women). Protein:creatinine ratio can also be used for monitoring in nondiabetes (it does not detect microalbuminuria, normal range: <15 mg/mmol). These tests ideally should use an early morning urine sample, although a ‘spot urine’ can be taken, then repeated with an EMU if positive. The urine should be kept refrigerated following the collection period to minimise bacterial growth. Twenty-four hour urine collections are no longer required for quantification of protein, however they may be required for other tests. See page 155.
Microalbuminuria
Microalbuminuria is defined as persistent small amounts of albumin not determined by the usual dipstick test and is of importance as a predictor of renal involvement in diabetes mellitus. The first sign of kidney damage due to diabetes is microalbuminuria, which can then proceed to larger quantities of protein excretion (proteinuria) and, possibly, low serum albumin, oedema, deranged blood chemistry and hypertension. Microalbuminuria is defined as persistent small amounts of albumin not determined by the usual dipstick test and is of importance as a predictor of renal involvement in diabetes mellitus. Whereas a normal sample of urine may contain albumin 2.5–25 mg/24 h, microalbuminuria is in the range 30–300 mg/24 h and macroalbuminuria is defined as greater than 300 mg/24 h (see Chapter 6).
Bence Jones protein
This test consists of a sample of urine from the first specimen of the day. In the laboratory the urine is heated; if this protein is present, it will precipitate on heating and dissolve at 100 °C; on cooling the protein will precipitate again. This test is now performed by electrophoresis and immunoelectrophoresis. It is most commonly (70–80% of positive results) found in multiple myeloma due to the proliferation of paraprotein-producing bone marrow. It is also occasionally seen in amyloidosis, cryoglobulinaemia and hyperparathyroidism.
Myoglobin levels
Myoglobinuria may occur due to conditions such as rhabdomyolysis where there is a breakdown of muscle tissue, causing the release of myoglobin into the blood stream. This may occur due to trauma or crush injury, seizures, immobility or severe exercise. The kidneys filter the blood and excrete the myoglobin in the urine. Myoglobin is nephrotoxic and large amounts of this protein can cause the occlusion of the renal tubules, leading to acute tubular necrosis and acute kidney injury. This can be diagnosed by a sample of urine – usually the first sample of the day.
Urine microscopy and culture
Microscopy will reveal information from the sediment found in urine – casts, crystals, blood cells and bacteria. The site of origin of casts can often be determined, indicating the type and extent of damage to the kidney. It is normal to find red blood cells in urine at approximately < 2 hpf and these normally originate from the renal pelvis, ureter or bladder and are uniform in shape and size. Leukocytes are also present at approximately < 2 hpf but the presence of eosinophils can be indicative of an allergic interstitial nephritis.
Casts
Different types of casts found in the urine may indicate underlying conditions. Hyaline casts, known as Tamm–Horsfall proteins, originate from the renal tubules. They may be present due to the use of diuretics, fever and exercise; however, they may also be present in kidney disease (for example, pyelonephritis). Granular or cellular casts may be seen in renal parenchymal disease. Red cell casts indicate bleeding and white cell casts indicate pyelonephritis or renal parenchymal disease.
If microorganisms are found to be present in the urine specimen – usually determined by the Gram staining method – the laboratory will provide information as to which antibiotic is most appropriate. The presence of multiple organisms may indicate contamination of the sample. It is important that the urine sample is collected before a broad-spectrum antibiotic is taken – this may be given in the interim period before specific sensitivity is ascertained.
A positive microscopy sample shows at least >105 cfu/ml and organisms 1–10 hpf; below this figure is not considered significant. However, with very dilute urine, a false-negative result may occur despite infection being present.
Kidney Function Tests
In CKD, a regular assessment of kidney function can be useful in monitoring the decline in kidney function and in predicting the time when RRT is likely to be needed. Glomerular filtration rate (GFR) tends to decrease in a linear fashion over time in progressive kidney disease and so, by extrapolation, predictions can be made as to when ESKD may occur. Measuring GFR provides details of the filtration rate of the functioning nephrons.
Knowledge of the amount of nephron damage is useful in assisting in making the choice of suitable drug regime. When there is more than 30% of nephron loss, certain drugs should be avoided or used with caution because of the slow excretion of the drug or its metabolites. GFR often declines with age (as much as 1 ml/min each year), starting at the fourth decade.
- Increased glomerular filtration rate can occur with:
- increased protein intake;
- diurnal variation;
- pregnancy.
- increased protein intake;
- Decreased glomerular filtration rate can occur with:
- exercise;
- age;
- low-protein diet;
- liver disease.
- exercise;
Many research studies and clinical trials of pharmaceuticals depend on regular glomerular filtration rate calculations to monitor the effect of treatments with regard to kidney function.
Kidney function investigations discussed here include creatinine clearance, the 51chromium ethylenediaminetetraacetic acid glomerular filtration rate, (51Cr EDTA GFR) and estimated glomerular filtration rate (eGFR).
eGFR (estimated glomerular filtration rate)
The eGFR (calculated from serum creatinine results) provides the best overall assessment of the level of kidney function. However creatinine clearance estimation (using 24 urine collection) can be helpful in the following situations:
- Estimation of GFR in individuals with exceptional dietary intake (vegetarian diet, creatine supplements) or decreased muscle mass (amputation, malnutrition, muscle wasting).
- Assessment of diet and nutritional status.
The eGFR can be calculated in adults using equations that take into account the serum creatinine concentration and some or all of the following variables: age, gender, race, and body size:
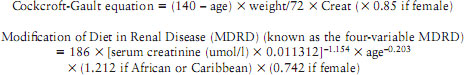
For adults the preferred equation is the MDRD (which uses creatinine, age, gender and ethnicity in its calculation). eGFR should not be used in AKI, as serum creatinine level may reflect a normal range as a result of delay in the decline in eGFR and the steady state of creatinine. eGFR should not be used in those <18 and >90 years or in pregnancy (National Institute for Health and Clinical Excellence 2011). Prior to measuring eGFR patients should be advised to not eat meat for 12 h before the test and for the most accurate results, the test should be processed within 12 h.
There is a tendency for the MDRD calculation to overestimate CKD stage 1 and 2. A newer method of measuring eGFR is the chronic kidney disease epidemiology collaboration (CKD-EPI) equation which has found in initial studies to be more accurate if GFR > 60 ml/min/1.73m2 (Matsushita et al. 2012).
Results and interpretation of eGFR calculations are discussed in Chapter 6.
Measurement of protein excretion
Twenty-four hour protein estimations are no longer recommended. The total daily protein excretion (in mg) can be estimated simply by multiplying the total protein-creatinine ratio (TPCR) (from a spot urine sample, preferably early morning, measured in mg/mmol) by a factor of ten. For example: Urine protein = 750 mg/l, urine creatinine = 7.5 mmol/l. So, total protein-creatinine ratio (TPCR) = 750/7.5 = 100 mg/mmol.
Therefore daily protein excretion = 100 × 10 = 1000 mg, or 1 g
Creatinine clearance
Note: this test is not recommended to estimate kidney function however may still be requested by the medical staff. The principle of clearance is that an estimation of a known substance in the plasma is compared with the amount in the urine. This substance must only be excreted in the urine. The calculation by which the clearance of the substance occurs can be measured is thus:

Because creatinine is believed to be manufactured at a fairly constant rate by the muscle mass, is circulating in the blood stream and is filtered by the glomeruli (although a very small amount is excreted by the tubules), this is the usual substance measured. When used in the above example, this is known as creatinine clearance.
About 50% of nephrons will have lost their function before an appreciable alteration occurs in the result of the creatinine clearance test. The normal value of creatinine clearance should be between 70 and 125 ml/min; the function lessens with age. A creatinine clearance result of less than 10 ml/ min is often an indication to start RRT.
Procedure
A 24h urine collection is made which will provide the urinary creatinine content (U) and volume (V). A blood sample should be taken to indicate the plasma creatinine (P).
Patient information for 24-h urine collection
The reason for the test should be explained to patients and they should be told what is expected of them. One (or more) 2 l collection bottles containing no additives or preservatives should be given to the patient. While male patients can usually void straight into the bottle, female patients should be provided with a suitable receptacle in which they can catch the urine. The patient must be instructed to discard the first urine of the day (on day 1) into the lavatory and then collect all urine passed for the next 24 h into the bottle provided. On the following morning (day 2), patients should empty their bladders precisely 24h after the initial sample and then the collection is complete. The completed urine collection should be labelled with the date and time of start and completion of the collection as well as the usual details such as name, identity number and date of birth.
The urine collection and the blood sample should be delivered to the laboratory with the request form, which should specify creatinine clearance test. The above formula is then applied and the creatinine clearance calculated.
CKD 51Chromium EDTA GFR
A more accurate method of assessing kidney function than the creatinine clearance test is the 51Cr EDTA GFR. As with the creatinine clearance test, the normal range is a clearance of 70–125 ml/min.
Patient preparation
The patient should be informed of the reason for this test, the fact that a small dose of a radioisotope will be injected, and the necessity of a series of blood samples over a 4 h period, and consent should be sought.
Procedure
The radiolabelled substance is given by intravenous injection. The patient’s weight and height must also be recorded to enable the result to be normalised for the individual patient’s body surface area.
Over the 4 h following the injection the usual procedure is for four blood samples to be drawn from the opposite arm to the injection of the radioisotope. This is to avoid contamination from any activity still lingering around the injection site, which will falsify the result.
Kidney Biopsy
Patients who are referred to the nephrology outpatient clinic with proteinuria, haematuria or renal impairment with no obvious cause may require a kidney biopsy in order that the nephrologist can make a diagnosis and commence appropriate treatment. Whilst in experienced hands kidney biopsy is a fairly safe procedure, there are risks which should be taken into consideration. Risks of kidney biopsy, which are greater in AKI, are perirenal haematoma, prolonged and severe bleeding necessitating blood transfusion and possible surgery, and rarely irreparable damage to the kidney requiring a nephrectomy.
Kidney biopsy is contraindicated in the following:
- small kidneys
- a single kidney
- cystic kidney or hydronephrosis
- UTI, acute pyelonephritis
- gross obesity
- uncontrolled hypertension
- nonadherent patient
- obvious diagnosis
- severe anaemia
- uncontrolled coagulopathy
Patient preparation
Information regarding the benefits and risks attached to this procedure should be given to the patient, who should be allowed the opportunity to ask questions and time to consider the implications before consenting to the biopsy. Children under 16 years usually need written parental consent.
Patients are usually admitted to the ward on the day planned for biopsy and a further explanation of the exact procedure and what is expected of the patient should be given prior to signature of a consent form. Children are fasted for 4 h before the biopsy as they will be sedated with a preparation such as midazolam following a mild premedication. In order to gain full compliance, whilst still in the ward, it is helpful to ask the patient to practise deep breathing and breath holding. Unless the patient can cooperate with breath holding on demand, the procedure should not be attempted, as the danger of malplacement of the sharp biopsy needle causing laceration or haemorrhage becomes a possibility.
Investigations include baseline urinalysis to exclude the presence of urinary tract infection, haemodynamic observations – the BP should be < 140/90 mmHg. Blood samples are required for FBC, group and save and clotting profile (Hb > 10 g/dl, INR > 1.2, bleeding time > 10 mins, platelets >100 × 109/l) as the highly vascular kidney can haemorrhage even when clotting times are within normal limits. It is also important to check if the patient is currently on any anticoagulant therapy (e.g. warfarin, heparin, clopidogrel), which should have been ceased five days prior to the procedure and that they are not taking any nonsteroidal anti-inflammatories or aspirin. Some patients may require DDAVP (a synthetic version of the antidiuretic hormone vasopressin which works as a haemostatic agent) (Mackie 2012) or a blood transfusion in the case of urea ≥ 20 mmol/l or creatinine ≥ 300 mmol/l or for abnormal clotting times. Check the patient is not allergic to iodine if to be used as antiseptic preparation solution.
Procedure
Patients will be asked to empty their bladders before the procedure. Percutaneous kidney biopsy may be performed on the ward or X-ray department, under local anaesthetic. The patient lies in a prone position, with a pillow under the upper abdomen to isolate the kidney, perhaps supported with sandbags to prevent movement. The kidney (usually left) is identified by real-time ultrasound as to position and depth and the skin is marked as to where the needle should be inserted. After cleaning the ultrasound gel from the skin, using a full aseptic technique, the area is cleaned, the area infiltrated with lidocaine as a local anaesthetic and a needle is inserted into the lumbar muscle layer until the needle is noted to swing with the patient’s respirations. The patient should be asked to hold the breath whilst the needle is advanced 5 mm at a time, leaving the needle to swing free when the patient breathes in and out. When the needle has located the kidney, more local anaesthetic should be injected.
The needle is then withdrawn, a small incision is made at the needle exit site and a Tru-cut kidney biopsy needle (or a spring loaded biospy gun) is inserted along the pathway made by the needle in the same manner, making advances as the patient holds the breath. When the kidney is again located, the biopsy is taken with the patient holding a breath. The tru-cut needle is withdrawn and the specimen obtained is immediately placed on a slide and viewed under a dissecting microscope to ascertain that cortex which has been obtained is large enough (about 5 mm length) to divide into three samples. If not enough cortex has been obtained, the tru-cut needle will have to be inserted again until a suitable strip of cortex containing sufficient glomeruli has been identified. Samples are sent to the laboratory for histology (in a 10% formalin pot), for immunofluorescence (in sterile normal saline) and electron microscopy (in specific glutaraldehyde fixative, kept cold). These samples should be delivered immediately (within minutes, not hours) to the laboratory, which must have had advance warning of the biopsy.
Finally, after the tru-cut needle has been withdrawn, a pressure dressing is applied and the patient is asked to remain flat in bed. The patient will need much encouragement and reassurance during the kidney biopsy procedure as it can be painful, despite local anaesthetic. A friendly hand to hold and quiet encouragement to cooperate with breathing requirements from the attending nurse can be very reassuring.
Patient care following kidney biopsy
It is usual practice to keep patients in lying flat for 2–6 h and bed rest will be dependent on unit policy, but it is common to be performed as a day case unless there is any frank haematuria which will require overnight observation. Haemorrhage and haematoma are the main complications following kidney biopsy; the wound site should be frequently checked for surface bleeding and blood pressure and pulse observations should be carried out until stable, for example on the time scale of every 15 min for 2 h, then every 30 min for 2 h, and then hourly for 4 h. The signs and symptoms giving an indication of internal bleeding are a rise or fall in blood pressure and dull aching pain in the abdomen, back or shoulder. The patient should be warned that some degree of haematuria will occur initially, but only persisting or heavy haematuria is of significance (1% require blood transfusions). Small urine samples from each void should be retained in transparent specimen containers for observation of diminishing haematuria and dipstick testing. The patient should be advised not to do any strenuous activity for 4 weeks, to check the puncture site for signs of swelling, bleeding and redness and contact the renal unit if they have any back pain, fever, dizziness or haematuria post biopsy.
Kidney biopsy in the transplant recipient
Closed percutaneous biopsies of the transplanted kidney are undertaken to support evidence of rejection and also to confirm suspicion of recurrent or primary glomerular disease (see Chapter 10). The procedure is similar to the biopsy of the native kidney but more straightforward due to the superficial position of the transplanted kidney. The patient will be placed in a supine position with a pillow beneath the transplant side to move the intraabdominal contents away from the site. The amount of tissue required in a transplant biopsy will be less than in a native kidney biopsy as fewer tests will be performed. The patient should remain resting in bed for 4–6 h and usually discharged home the same day, though it is important to ensure the patient has passed urine and a dipstick test has been performed to check for blood.
Radiographic Investigations
Investigations using various radiographic methods are often employed to assist diagnosis and to assess progression of kidney disease and its attendant side effects. The most common techniques are discussed here.
Patients should have received adequate explanations before entering the department in order to allay any fears they may have on finding themselves in a department full of strange machinery, hazard warnings and unfamiliar staff. If they are aware of the reasons for the investigation and what will be expected of them, the likelihood of an accurate result of the examination will be enhanced. The patient will be asked to sign a consent form for some invasive tests and early information will be of help for understanding the procedure.
All investigations involving X-rays must be performed according to the safety regulations in using a potentially hazardous substance, and these techniques must not be used unless the risk to the patient is outweighed by the benefit. It is important that women of child-bearing years are asked specifically if there is any possibility of pregnancy prior to carrying out the test. The foetus is most vulnerable to ionising radiation in the first trimester and the woman may not know that she is pregnant, so if there is any possibility it should be discussed with the referring doctor.
Plain abdominal X-ray
Plain abdominal X-rays incorporating the kidneys, ureters and bladder (KUB) indicate the size, shape, position and the presence or absence of one or both kidneys, and may be taken before other more complicated radiological procedures in order to provide an overall background picture. Most calculi may be seen as they are usually composed of radiopaque material. KUB X-rays are usually taken from the anterior aspect. A combination of KUB and ultrasound often forms the basic routine screening in those with kidney disease.
Bone density scan
Also known as dual energy x-ray absorptiometry (DEXA) scan measures the mineral density of bones using a low dose noninvasive x-ray technique. The x-ray beam has two energy peaks, one aimed at the bones and the other the tissue, then the tissue amount is subtracted providing a bone mineral density amount which indicates the strength of the bones.
Skeletal X-rays
Skeletal X-rays are not commonly peformed as superseded by bone density scans, but may be taken in the patients on dialysis. This is to detect osteodystrophy, which may become apparent in association with impaired glomerular filtration and associated disturbed metabolism of calcium and phosphate. Those bones most likely to show the characteristic abnormalities are the phalanges, skull, pelvis and vertebrae. Pain and deformity will ultimately develop unless imbalances of calcium and phosphate can be corrected and inadequate metabolism of vitamin D can be halted (see Chapter 13).
Intravenous urogram (IVU)
This procedure is also known as the intravenous pyelogram (IVP). This examination indicates the size and position of the kidneys and the anatomy of the calyces and pelvis. The ureters are also outlined by the progression of the dye containing urine to the bladder and the subsequent use of sequential X-rays, enabling any deformities in these organs to be demonstrated. The IVU gives little useful information in advanced CKD and consequently is not the investigation of choice if more than 50% of nephron loss is suspected. If impaired kidney function is known and an IVU is indicated, a greater dose than usual of the radiopaque contrast medium may need to be given – this in itself is nephrotoxic and may exacerbate CKD, at least temporarily.
Patient preparation
The patient should be told that the investigation will take about an hour to complete – longer if there is renal impairment. If the patient is taking metformin it must be stopped 24 h prior to prevent the risk of lactic acidosis. After an explanation of the procedure with adequate time to ask questions, the patient may be asked to sign a consent form and be checked for any allergies to iodine or shellfish. Caution should be observed with asthmatics and others who have allergic conditions, as the contrast medium is iodine based. Therefore, it is standard procedure that injections of adrenaline (epinephrine) 0.5–1 mg (0.5–1 ml of 1:1000 solution = 1 mg/ml) i.m., antihistamine (e.g. chlorphenamine (chlorpheniramine) 10–20 mg i.v.) and hydrocortisone should be immediately available to treat anaphylaxis should it occur. Some patients may require prophylactic corticosteroids if there is a history of atophy or asthma.
Bowel preparation will be dependent on local policy and, if used, should be carried out two days prior to the procedure to clear the bowel to enable a clear view of the urinary system. It may not be used in older patients as there is a risk of dehydration, which could lead to reduced kidney perfusion. Adult patients should fast for 4–6 h, but limited clear fluid is usually allowed until 1 h before the IVU. Emptying the bladder beforehand is important, or the contrast will become overdilute on reaching a full bladder and a poor picture will result. A fluid restriction of 500 ml in 24 h may be requested if the patient has normal kidney function for better images as a result of concentrated contrast media.
Post procedure, kidney function should be checked in known CKD and urinalysis checked for any sign of haematuria. Haemodynamic observations should checked for signs of infection or bleeding and allergic reaction to the contrast agent. Symptoms of mild to moderate allergy include pruritis, urticaria and vomiting, and can be treated with an antihistamine. Symptoms of severe anaphylaxis (a medical emergency) include bronchospasm, hypotension and shock. Any reaction should be noted in the medical file for any further procedures. The patients should be encouraged to drink fluids if not contraindicated, to aid clearance of the contrast media. Nurses should be aware that there is a possibility of acute kidney injury following this investigation, so urinary output should be monitored. Prior to restarting metformin, a check creatinine level is required 48 h post procedure.
Retrograde pyelogram
In this examination, radio–opaque dye is injected directly into the upper urinary tract via a catheter inserted through a cystoscope into the ureter. A series of X-rays are performed on one or both kidneys. This test is useful in outlining stones, calyceal defects and masses in the ureter or renal pelvis and in defining deformities such as hydronephrosis or hydroureter. This investigation is sometimes performed after an IVU or ultrasound (US) has demonstrated a hydronephrosis and more clarification is needed for a diagnosis. After the procedure the urine should be observed for haematuria and patients should be watched for signs and symptoms of infection. Post procedure care is as for IVU. Patients should be encouraged to drink copiously to help avoid infection, unless contraindicated due to reduced fluid allowance (antibiotics may be given as a prophylaxis).
Computed axial tomography or computed tomography (CAT/CT) scan
This investigation is reserved for the patient who needs staging of a renal mass or a diagnosis when other methods of detection have failed to provide a clear picture. Computer tomography is an X-ray technique that uses a computer to reconstruct cross-sectional images of 1 cm slices of the organ targeted. The dose of radiation is about the same as that for an IVU. A clear bowel is necessary so a suitable laxative may be given 2 days before the scan. A light diet should be taken for 2 days before the scan and nothing on the day of examination apart from clear fluids. Patients must be able to follow instructions such as when to hold the breath, to be able to lie motionless and not to talk. Contrast agents may be used to assess the renal cortex better, so check for any allergy to contrast media or history of asthma.
Nuclear magnetic resonance or magnetic resonance imaging (MRI)
This form of scanning involves application of a strong external magnetic field along with a radiofrequency signal that produces a current in a receiving coil proportional to the density of protons in the body organ being scanned. This signal is processed by computer to create a tomographic slice of the organ similar to a CT scan. In renal medicine a clear picture of tumour invasion into blood vessels can be demonstrated as well as differentiation of tissue character.
The advantage of MRI over CT imaging is that no ionising radiation is used. A non-nephrotic contrast agent may be used and many planes can be visualised. Caution is required with the use of gadolinium as it has been associated with nephrogenic systemic fibrosis and kidney function should be checked prior to use. Older patients are more at risk and high risk gadolinium should not be used in severe kidney disease (European Medicines Agency 2010).
It is important that the patient is not wearing any magnetic metal object, and it must be ascertained that no internal metal objects are present such as aneurysm clips, screws, pacemakers or shrapnel.
Ultrasound
Ultrasound investigations have replaced some x-ray procedures (especially the IVU) to a large degree, and because this procedure does not carry the hazards associated with radiation this method can be used in women without consideration of the possibility of pregnancy. This is a noninvasive procedure where a transducer (sonar probe) is moved in close contact with the skin over the area of investigation and it can be repeated frequently if necessary, unlike X-ray. Ultrasound is especially useful in examinations of the abdominal and pelvic organs. It is widely used to determine the size and shape of the kidney, its presence and position, and the composition of cysts or neoplasms if present, and also in the diagnosis of polycystic kidney disease. However, it is less useful in providing information about the ureters. Ultrasound is also used to guide the operator in procedures such as kidney biopsy.
Patients who are to have kidney US scans are usually asked to fast for 6–8 h (in order to keep pockets of air in the gut to a minimum) except for drinks of clear fluids.
Ultrasound in kidney transplantation
The most important and common cause of early transplant dysfunction is acute rejection, which occurs in 10–20% of all patients. This is accompanied by inflammation, which leads to swelling of the kidney and an increase in pressure inside the organ. Ultrasound can visualise any changes to the kidney and can be undertaken daily and two size increases on consecutive days would be strongly indicative of acute rejection. However, dysfunction attributed to other causes must be excluded. Infection may be associated with an increase in the kidney size, but this is easily diagnosed by routine testing of midstream specimens of urine.
Renal vein thrombosis is a serious complication and will cause a rapid increase in size, possibly resulting in a tear of the kidney substance. If this condition is quickly diagnosed with US, rapid surgical intervention is possible and the graft may be rescued. Renal artery thrombosis may be diagnosed ultrasonically by the observation of lack of vessel pulsation. For greater accuracy, duplex scanning using a combination of imaging and frequency waveform analysis is available.
Early complications following transplantation include urine leaks, usually from the site of the ureteric anastomosis. These are seen ultrasonically as a fluid collection around the graft site. Later complications are obstructive lesions, which are often insidious in onset and lead to deteriorating kidney function. Routine scanning of outpatients is a simple and easy method of detecting dilatation and stenoses of the urinary tract, which are then treated surgically.
Needle core biopsy of the transplanted kidney remains the best method of detecting rejection and determining the degree of interstitial fibrosis and acute inflammation. Using ultrasound guidance of the biopsy needle, a good core of the renal cortex may be safely obtained, carefully avoiding the structures of the renal medulla and damage to the graft. Ultrasound is therefore a valuable tool in the detection and diagnosis of kidney allograft dysfunction, allowing intervention and early treatment of problems.
Renal doppler studies
A noninvasive ultrasound test to assess vascular flow to the kidney, for example renal vein thrombosis, acute tubular necrosis, renal artery stenosis and evaluate transplanted kidneys for urine outflow obstruction and acute rejection.
Renograms
An injection of intravenous radioistope, which is excreted via the kidneys, and a series of x-rays are taken over a 2-4 h period, which enables the assessment of kidney function and tubular secretion and can indicate the position of an obstruction – no radioistope is seen if there is a reduction in blood supply to the kidney or in the obstructed area. This investigation can be used in place of IVU if the patient is allergic to iodine contrast medium, and it is also used in transplanted patients. In renal artery stenosis, a renogram (using either DPTA or MAG 3) may be performed and then repeated, incorporating an injection of captopril as a test dose to outline any response induced – i.e. a reduction in renal blood flow in the presence of an blockage. Check with medical staff if the patient on antihypertensive treatment as he/she may need to stop or adjust dose. Blood pressure should be monitored after the procedure for 2 h.
Renal scans
There are three types of radioisotope scan that can be performed to provide quantitative data on the function of the kidneys.
Firstly, the isotope, 99mtechnetium-labelled diethylenetriaminepentaacetic acid (99mTc DTPA), is rapidly excreted by the kidney and shows the blood flow through the kidneys, identifies obstructions, for example renal artery stenosis, and provides valuable information about the function and excretion capacity of the kidney. A diuretic may also be given intravenously and the patient should be well hydrated. The procedure takes about 1 h. It is not as effective if creatinine > 200 μmol/l.
The second isotope is 99mTechnetium-labelled dimercaptosuccinic acid (99mTc DMSA) which is retained by the cells in the proximal tubules and parenchyma and enables the identification of areas of cortical scarring, contusions and solid lesions. This will provide quantification of relative kidney function between kidneys and within a kidney.
The third is known as MAG 3, which uses the isotope 99mtechnetium-labelled benzoylcaptoacetyltriglycerine and is a dynamic imaging scan as the isotope is rapidly excreted by the kidney via glomerular filtration or a combination of filtration and tubular secretion. The test enables visualisation of the aorta and renal perfusion. Quantification of renal blood flow can be calculated and it identifies the overall kidney function and presence of obstruction, thrombus, emboli and stenosis. When patients undergo radioisotopic scans they should empty their bladders immediately before scanning. Patients should be advised that they are radioactive for 24 h and nursing staff should ensure universal precautions when managing waste disposal.
Renal angiogram
This is performed through a catheter inserted via the femoral vein (however the brachial artery may also be used if required) and fed to the renal artery. Radiopaque contrast dye is injected and a series of fluoroscopic x-rays performed. It identifies tumours, emboli, thrombosis, trauma and stenosis of vessels and for transplant donor assessment and identification of bleeding site post kidney biopsy. Caution should be taken with patients who have some degree of renal insufficiency as the dye is nephrotoxic. If patients are able then they should drink plenty of fluids to flush through the contrast.
The most common complication of a femoral angiogram is bruising, false aneurysm, haemorrhage, infection, reaction to the contrast agent and haematoma. Consent is required.
The patient should fast 4 h for food and 2 h for clear fluids prior to the procedure and must be checked for any allergies to iodine and shellfish. It is important to ensure that hydration is adequate, especially if there is decreased kidney function and low urine output, which may require the use of a nephroprotective agent or IV 0.9% normal saline or 1.26% sodium bicarbonate over a 4 h period prior. Other agents used include N-acetylcysteine (NAC) for low urine output and impaired kidney function. Anticoagulants may be required (if INR < 1.7) and possibly heparin. Metformin should be stopped preprocedure to prevent lactic acidosis. Baseline neurovascular observations should be recorded. Bloods samples should be taken for FBC, U&Es and clotting profile.
After the procedure, the frequency of observations of the puncture site, neurovascular and haemodynamic observations are dependent on the unit policy, but as a guide should be performed every 15 min for 1 h, then half-hourly for 2 h for signs of bleeding whilst the patient is lying flat or at 45 degrees for 1–2 h and bed rest for up to 24 h, which is dependent on unit policy. Increase oral intake where possible to flush contrast media. Kidney function should be checked if known renal impairment and serum creatinine checked 48 h post recommencement of metformin. Anticoagulants should be recommenced, usually the evening postprocedure.
Patients should be advised not to do any strenuous exercise for 48–72 h or have a bath/shower for 24 h. The puncture site should be patted dry and slight pressure placed over the site initially if the patient coughs or sneezes.
Summary
The investigations which have been discussed above are by no means exhaustive. Since those with kidney disease usually have multifactorial disease processes many other specific investigations may be indicated, especially cardiovascular tests such as electrocardiography and echocardiography. The gastrointestinal tract in patient with kidney disease are frequently investigated for bleeding problems using techniques involving endoscopy (e.g. gastroscopy, colonoscopy).
Such is the commercial pressure to exploit the latest technology, it is inevitable that new methods and procedures will enter the renal field in the near future, condemning some present-day tests to redundancy. However, it is hoped that if nurses understand something of the current techniques used in renal investigations, they will be able to inform and reassure their patients reliably.
References
Department of Health. Guidance for Clinical Health Care Workers (1998) Protection Against Infection with Blood-borne Viruses – Recommendations of the Expert Advisory Group on AIDS and the Advisory Group on Hepatitis, HMSO, London.
European Medicines Agency (2010) Questions and Answers on the Review of Gadolinium Containing Contrast Agents, London, EMA, www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Gadolinium-containing_contrast_agents/human_referral_000182.jsp&mid=WC0b01ac0580024e9a (accessed 20 May 2013).
Fluck, R and Kumwenda, M. (2011) Renal Association Clinical Practice Guidelines: Vascular Access for Haemodialysis, 5th edn, Renal Asssociation, London.
Health and Safety Executive (HSE) (2010) Consultation on Proposals to Implement the 2010 Sharps in Healthcare Directive. www.hse.gov.uk/aboutus/meetings/hseboard/2012/270612/pjb1251.pdf (accessed 20 May 2013).
Health and Safety Executive (HSE) (2013). Health and Safety (Sharps Instruments in Healthcare) Regulations 2013: Guidance to Employers and Employees – information sheet, www.hse.gov.uk/pubns/hsis7.htm (accessed 24 May 2013.)
Jun, M., Lv, J., Perkovic, V. and Jardine, M.J. (2011) Managing cardiovascular risk in people with chronic kidney disease: a review of the evidence from randomized controlled trials. Therapeutic Advances in Chronic Disease 2 (4), 265–278.
Locatelli, F, Pozzoni, P. and Del Vecchio, L. (2007) Recombinant human epoetin beta in the treatment of renal anaemia. Therapeutics and Clinical Risk Management 3 (3), 433-439.
Mackie, S. (2012) An overview of percutaneous renal biopsy. Journal of Renal Nursing, 4 (2), 90–95.
Matsushita, K., Mahmoodi, B.K., Woodward, M. et al. (2012) Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. Journal of the American Medical Association 307 (18), 1941–1951.
National Institute for Health and Clinical Excellence (2008) Clinical Guideline 73. CKD: Early Identification and Management of CKD in Adults in Primary and Secondary Care, National Institute for Health and Clinical Excellence, London.
National Institute for Health and Clinical Excellence (2010) Clinical Guideline 67. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for Primary and Secondary Prevention of Cardiovascular Disease, National Institute of Health and Clinical Excellence, London.
National Institute for Health and Clinical Excellence (2011) Clinical Guideline 114: Anaemia Management in Chronic Kidney Disease: National Clinical Guideline for Management in Adults and Children, Royal College of Physicians, London, www.nice.org.uk/guidance /CG114 (accessed 20 May 2013).
Nursing and Midwifery Council (1992) Perceptions of the Scope of Professional Practice, UKCC, London.
Williams, J.D. and Phillips, A.D. (2010) Section 21: Disorders of the kidney and urinary tract. In Oxford Textbook of Medicine (eds A. Warrell, T. Cox and D. Firth), 5th edn, Oxford University Press, Oxford.
World Health Organization (WHO) (2010) WHO Guidelines on Drawing Blood: Best Practice in Phlebotomy. WHO Safe Injection Global Network (SIGN), WHO, Geneva.
Useful web sites
BC Renal Agency, Vascular Access Guideline. Chronic Kidney Disease: Vein Preservation, March 2011, revised August 2012, www.bcrenalagency.ca/NR/rdonlyres/AC0BB28B-00A9-41A6-BD76-55F441559B03/59299/VeinPreservationRenalPatientsUpdateAug172013.pdf (accessed 20 May 2013).
EdrenINFO, www.edren.org/pages/edreninfo/ckd-chronic-renal-failure-and-its-progression.php (accessed 20 May 2013).
Lab Tests On-line, www.labtestsonline.org.uk/ (accessed 20 May 2013).
Royal College of Radiologists, www.rcr.ac.uk (accessed 20 May 2013)
The UK Renal Association. About eGFR, www.renal.org/whatwedo/InformationResources/CKDeGUIDE/AbouteGFR.aspx (accessed 20 May 2013).
UK Renal Association. Patient Information Leaflets for Kidney Biopsy (Native and Transplanted), Angioplasty and Stent Insertion, www.renal.org/whatwedo/informationresources/patients.aspx (accessed 20 May 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


