Chapter 15 Infusion Therapy*
Safe and Effective Care Environment
1. Prevent IV administration errors by following best practices that ensure patient and staff safety.
2. Explain how emerging technology related to infusion therapy can enhance patient safety.
3. Teach the patient and family about the type and care related to the patient’s infusion therapy.
4. Identify the evidence-based recommendations for prevention of intravenous (IV) catheter–related bloodstream infection (CR-BSI).
Health Promotion and Maintenance
6. Check the accuracy of prescriptions for IV fluids and drug therapy.
7. Identify the appropriate veins for peripheral IV catheter insertion.
8. Differentiate types of vascular access devices (VADs) used for peripheral and central IV therapy.
9. Use best practice for inserting peripheral vascular access devices (VADs).
10. Assess the patient’s infusion site frequently for local complications, such as phlebitis and infiltration.
11. Prioritize nursing interventions for maintaining an infusion system.
12. Assess, prevent, and manage systemic complications related to infusion therapy and VADs.
13. Identify nursing care associated with intra-arterial, intraperitoneal, subcutaneous, intraosseous, and intraspinal infusion therapy.
http://evolve.elsevier.com/Iggy/
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Review Questions for the NCLEX® Examination
Overview
• Maintain fluid balance or correct fluid imbalance
• Maintain electrolyte or acid-base balance or correct electrolyte or acid-base imbalance
Having a specialized team of infusion nurses to initiate and maintain infusion therapy is recommended by the Centers for Disease Control and Prevention (CDC) to reduce complications of infusion therapy (O’Grady et al., 2011). These teams have demonstrated value in cost savings, patient satisfaction, and patient outcomes.
Infusion nurses may perform any or all of the following activities:
• Develop evidence-based policies and procedures
• Insert and maintain (sterile dressings and flushing) several types of peripheral and central venous catheters
• Educate staff, patients, and families
• Consult on product selection and purchasing decisions
• Therapies such as blood withdrawal, therapeutic phlebotomy, hypodermoclysis, intraosseous infusions, and administration of medications including chemotherapy
The registered nurse (RN) generalist is taught to insert peripheral IVs, and most institutions have a process of credentialing this skill. Depending on the state’s nurse practice act, licensed practical/vocational nurses (LPNs/LVNs) and technicians can be trained and credentialed to perform the skill of peripheral IV insertion and assist with infusions, but the RN is ultimately accountable for all aspects of infusion therapy and delegation of associated tasks (Infusion Nurses Society [INS], 2006).
Types of Infusion Therapy Fluids
Many types of parenteral fluids are used for infusion therapy. These fluids include:
Intravenous Solutions
More than 200 IV fluids (solutions) are available that meet the requirements established by the United States Pharmacopeia (USP). Each solution is classified by its tonicity (concentration) and pH. Tonicity is typically categorized by comparison with normal blood plasma as osmolarity (mOsm/L). As discussed in Chapter 13, normal serum osmolarity for adults is between 270 and 300 mOsm/L. Parenteral solutions within that normal range are isotonic; those fluids greater than 300 mOsm/L are hypertonic; and those fluids less than 270 mOsm/L are hypotonic. Table 13-4 in Chapter 13 lists common IV solutions according to their tonicity.
When an isotonic infusate (solution that is infused into the body) is used, water does not move into or out of the body’s cells. Therefore patients receiving isotonic solutions are at risk for fluid overload, especially older adults. This complication is discussed in Chapter 13. Hypertonic fluids are used to correct fluid, electrolyte, and acid-base imbalances by moving water out of the body’s cells and into the bloodstream. Parenteral nutrition solutions are also hypertonic (see Chapter 63). Instead of moving water out of cells, hypotonic infusates move water into cells to expand them. Patients receiving either hypertonic or hypotonic fluids are at risk for phlebitis and infiltration. Phlebitis is the inflammation of a vein caused by mechanical, chemical, or bacterial irritation. Infiltration occurs when IV solution leaks into the tissues around the vein.
The pH of IV solutions measures the acidity or alkalinity and usually ranges from 3.5 to 6.2. Extremes of both osmolarity and pH can cause vein damage leading to phlebitis and thrombosis (blood clot in the vein). Thus fluids and medications with a pH value less than 5 and more than 9 and with an osmolarity more than 600 mOsm/L are best infused in the central circulation where greater blood flow provides adequate hemodilution (Perucca, 2010). For example, total parenteral nutrition (TPN) solutions have an osmolarity greater than 1400 mOsm/L. TPN should not be infused in peripheral circulation because it can damage blood cells and the endothelial lining of the veins.
Drug Alert
Drugs such as amiodarone (Cordarone), vancomycin (Vancocin), and ciprofloxacin (Cipro I.V.) are venous irritants and have a pH less than 5. Phlebitis occurs when patients require long-term infusion of these drugs in peripheral circulation. Drugs with vasoconstrictive action, such as dopamine, or chemotherapeutic agents, such as vinblastine, are vesicants that can cause extravasation. Extravasation results in severe tissue damage as manifested by blistering, tissue sloughing, or necrosis from infiltration into the surrounding tissues (see Complications of Intravenous Therapy section on p. 227 for further explanation and Chapter 24 for more detail). Monitor the IV insertion site carefully for early manifestations of infiltration, including swelling, coolness, or redness. If any of these symptoms are present, discontinue the drug immediately and notify the infusion therapy team, if available. If an infusion specialist is not available, plan to remove the IV catheter and consider central line placement.
Blood and Blood Components
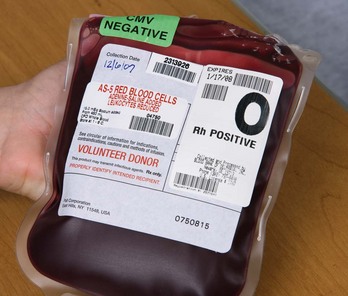
Blood transfusion is given by using packed red blood cells, created by removing a large portion of the plasma from whole blood. Other available blood components include platelets, fresh frozen plasma, albumin, and several specific clotting factors. Each component has detailed requirements for blood-type compatibility and infusion techniques. For patient safety, The Joint Commission’s 2010 National Patient Safety Goals (NPSGs) require agencies to ensure that blood components are properly ordered, handled and dispensed, and administered and that patients are appropriately monitored. Positive patient identification using two patient identifiers and requiring two qualified health care professionals is essential before any blood or blood component is administered. Most organizations use the International Society of Blood Transfusion (ISBT) universal bar-coding system to ensure the right blood for the right patient (Fig. 15-1). The ISBT system includes four components that must be present on the blood label both in bar code and in eye-readable format. These four components are (1) a unique facility identifier, (2) the lot number relating to the donor, (3) the product code, and (4) the ABO group and Rh type of the donor.
An acute hemolytic transfusion reaction caused by an incompatible blood transfusion is a “sentinel event” requiring an intense analysis of the contributing factors and corrective action. Established policies and procedures must be rigidly followed to ensure a safe transfusion and reduce the risk for complications. (See Chapter 42 for a complete discussion of blood transfusion administration.)
Drug Therapy
Procedures must be established to prevent errors resulting from look-alike, sound-alike drugs such as Celebrex IV (celecoxib) and Cerebyx (fosphenytoin). Other strategies to reduce errors include limiting available concentrations of drugs and dispensing all drugs, including catheter flush solutions, in single-dose containers. Smart pumps, with drug libraries (see Infusion Systems section), in combination with computer physician order entry (CPOE) and barcode medication administration (BCMA) systems, use recent technology to help reduce adverse drug events (ADEs). Electronic medication administration records (MARs) and multiple checks by pharmacists, as required by The Joint Commission’s NPSGs, also help reduce errors.
Prescribing Infusion Therapy
• Rate of administration written in milliliters per hour, or the total amount of fluid and the total number of hours for infusion (e.g., 125 mL/hr or 1000 mL/8 hr)
• Drugs and the specific dose to be added to the solution, such as electrolytes or vitamins
A drug prescription should include:
• Drug name, preferably by generic name
• Purpose (required in some health care agencies, especially nursing homes)
Action Alert
Peripheral Intravenous Therapy
Short Peripheral Catheters
Short peripheral catheters are composed of a plastic cannula built around a sharp stylet extending slightly beyond the cannula (Fig. 15-2). The stylet (sharp) allows for the venipuncture, and the cannula is advanced into the vein. Once the cannula is advanced into the vein, the stylet is withdrawn. These catheters are designed with a safety mechanism to cover the sharp end of the stylet after it is removed from the patient. These stylets are hollow-bore, blood-filled needles that carry a high risk for exposure to bloodborne pathogens if needle stick injury occurs. A federal law enacted in 2000 amended the Bloodborne Pathogen Standards from the Occupational Safety and Health Administration (OSHA) requiring the use of catheters with an engineered safety mechanism to prevent needle sticks.
Insertion and Placement Methods
Short catheters range in length from 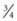 inch to
inch to 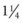 inch with gauge sizes from 26 gauge (the smallest) to 14 gauge (large bore). Choose the smallest gauge catheter capable of delivering the prescribed therapy. Current design allows for a thin-wall construction, providing a larger lumen (opening) without increasing the outer diameter. This design improves the fluid flow through the catheter while using a smaller gauge and thereby decreases the possibility of vein irritation from a large catheter. For example, a thin-walled 24-gauge Insyte (BD Medical) catheter has about the same flow-rate ability as a 22-gauge non–thin-walled Angiocath (BD Medical). Larger gauge sizes allow for faster flow rates but also cause phlebitis more often. Table 15-1 lists each gauge size and its common uses.
inch with gauge sizes from 26 gauge (the smallest) to 14 gauge (large bore). Choose the smallest gauge catheter capable of delivering the prescribed therapy. Current design allows for a thin-wall construction, providing a larger lumen (opening) without increasing the outer diameter. This design improves the fluid flow through the catheter while using a smaller gauge and thereby decreases the possibility of vein irritation from a large catheter. For example, a thin-walled 24-gauge Insyte (BD Medical) catheter has about the same flow-rate ability as a 22-gauge non–thin-walled Angiocath (BD Medical). Larger gauge sizes allow for faster flow rates but also cause phlebitis more often. Table 15-1 lists each gauge size and its common uses.
TABLE 15-1 CHOOSING THE GAUGE SIZE FOR PERIPHERAL CATHETERS
| CATHETER GAUGE | INDICATIONS | APPROXIMATE FLOW RATES |
|---|---|---|
| 24-26 gauge Smallest, shortest ( 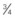 -inch length) -inch length) | Not ideal for viscous infusions Expect blood transfusion to take longer Preferred for infants and small children | 24 mL/min (1440 mL/hr) |
| 22 gauge | Adequate for most therapies, blood can infuse without damage | 38 mL/min (2280 mL/hr) |
20 gauge (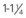 -inch length) -inch length) | Adequate for all therapies Most anesthesiologists prefer not to use a smaller size than this for surgery cases | 65 mL/min (3900 mL/hr) |
| 18 gauge | Preferred size for surgery Vein needs to be large enough to accommodate the catheter | 110 mL/min (6600 mL/hr) |
| 14-16 gauge | For trauma and surgical patients requiring rapid fluid resuscitation Needs to be in a vein that can accommodate it |
Short peripheral catheters are allowed to dwell (stay in) for 72 to 96 hours but then require removal and insertion at another venous site. If the patient’s therapy is expected to be longer than 6 days, a midline catheter or PICC should be chosen (O’Grady et al., 2011). When selecting the site for insertion of a peripheral catheter, consider the patient’s age, history, and diagnosis; the type and duration of the prescribed therapy; and, whenever possible, the patient’s preference. Chart 15-1 lists the major criteria for the placement of peripheral VADs.
Chart 15-1 Best Practice for Patient Safety & Quality Care
Placement of Short Peripheral Venous Catheters
• Verify that the prescription for infusion therapy is complete and appropriate for infusion through a short peripheral catheter.
• For adults, choose a site for placement in the upper extremity. DO NOT USE THE WRIST.
• Choose the patient’s nondominant arm when possible.
• Choose a distal site, and make all subsequent venipunctures proximal to previous sites.
• Do not use the arm on the side of a mastectomy, lymph node dissection, arteriovenous shunt or fistula, or paralysis.
• Avoid choosing a site in an area of joint flexion.
• Avoid choosing a site in a vein that feels hard or cordlike.
• Avoid choosing a site close to areas of cellulitis, dermatitis, or complications from previous catheter sites.
• Choose a vein of appropriate length and width to fit the size of the catheter required for infusion.
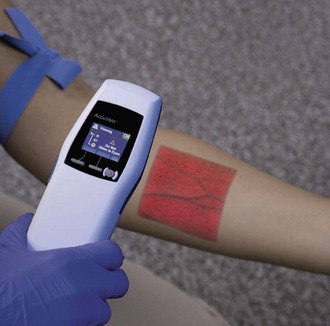
Though not yet widely used in most clinical settings, vein transilluminators and ultrasound devices are now available as tools to assist in IV line placement. Several different types of portable vein transilluminators are available, such as VeinViewer, Veinlite LED, and AccuVein (Fig. 15-3). Although they may have different mechanisms of action, some using infrared light and some using laser, these devices penetrate only up to approximately 10 mm and so are limited to finding superficial veins.
Site Selection and Skin Preparation
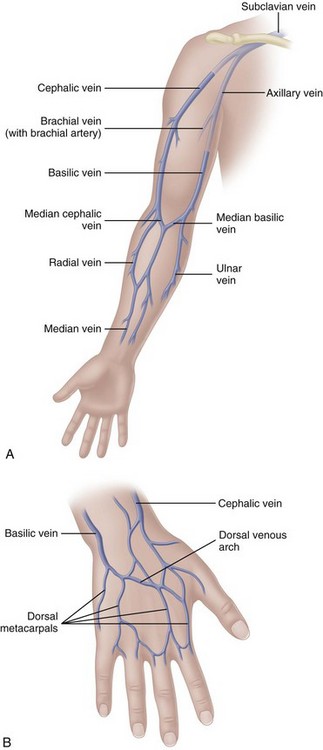
The most appropriate veins for peripheral catheter placement include the dorsal venous network, basilic, cephalic, and median veins, as well as their branches (Fig. 15-4). However, cannulation of veins on the hand is not appropriate for older patients with a loss of skin turgor and poor vein condition and for active patients receiving infusion therapy in an ambulatory clinic or home care. Use of veins on the dorsal surface of the hands should be reserved for short-term infusion of non-vesicant and non-irritant solutions.
Critical Rescue
Avoid veins on the palmar side of the wrist because the median nerve is located close to veins in this area, making the venipuncture more painful and difficult to stabilize. The cephalic vein begins above the thumb and extends up the entire length of the arm. This vein is usually large and prominent, appearing as a prime site for catheter insertion. However, the sensory branch of the median nerve can intersect with the cephalic vein up to three times from its origin to about 4 to 5 inches up the lateral aspect of the arm. Damage to the nerve can result in permanent loss of function or complex regional pain syndrome, type 2 (CRPS) (Masoorli, 2007). Reports of tingling, feeling “pins and needles” in the extremity, or numbness during the venipuncture procedure can indicate nerve puncture. If any of these symptoms occur, stop the IV insertion procedure immediately, remove the catheter, and choose a new site.
• Perform hand hygiene before palpating the insertion site.
• Wear clean gloves for peripheral IV insertion; do not touch the access site after application of antiseptics.
• Prepare clean skin with 70% alcohol (or chlorhexidine) before peripheral venous catheter insertion.
• Apply povidone-iodine to the skin, and allow it to dry for at least 2 minutes.
Midline Catheters
Midline catheters can be anywhere from 3 to 8 inches long, 3 to 5 Fr, and double or single lumen. They are inserted through the veins of the upper arm. The median antecubital vein is used most often if insertion is done without the aid of ultrasound guidance. With ultrasound guidance, deeper veins can be accessed and the insertion site can be further above the antecubital fossa. The basilic vein is preferred over the cephalic vein because of its larger diameter and straighter path. It also allows greater hemodilution of the fluids and medications being infused. The catheter tip is located in the upper arm with the tip residing no further into the venous network than the axillary vein (Fig. 15-5). These catheters are used for therapies lasting from 1 to 4 weeks; however, there are no recommendations for the optimal dwell time. Because of the extended dwell time, strict sterile technique is used for insertion and dressing changes for a midline catheter (Rosenthal, 2008). Additional education and skill assessment are required for the nurse to be qualified to insert midline catheters.
• Longer than 6 days and up to 4 weeks of therapy such as:
• Heparin infusions for deep vein thrombosis
• Bronchodilators, such as aminophylline
Midline catheters are considered to dwell in the peripheral circulation; the recommendations for infusates are the same as for short peripheral IVs. Fluids and medications infused through a midline catheter should have a pH between 5 and 9 and a final osmolarity of less than 600 mOsm/L (Perucca, 2010; Rosenthal, 2008). The pH and osmolarity outside these parameters increase the risk for complications like phlebitis and thrombosis. Midline catheters should not be used for infusion of vesicant medications—drugs that cause severe tissue damage if they escape into the subcutaneous tissue (extravasation). At a midline tip location, larger amounts of the drug can extravasate before the problem is detected.
Central Intravenous Therapy
In central IV therapy, the vascular access device (VAD) is placed in the central circulation, specifically within the superior vena cava (SVC) near its junction with the right atrium. Blood flow in the SVC is approximately 2 L/min compared with about 200 mL/min in the axillary vein. All central vascular access devices require confirmation of tip location by chest radiograph before solutions are infused (Hadaway, 2010a). However, the use of newer ultrasound systems, such as the Sherlock PICC tip location system, can decrease the need for repeated x-rays by confirming approximate catheter position. The practitioner inserts a specially designed magnetic stylet through the catheter before insertion into the patient so that the system can detect its tip location. These devices have been endorsed by the CDC and Agency for Healthcare Research and Quality (Moreau, 2008a).
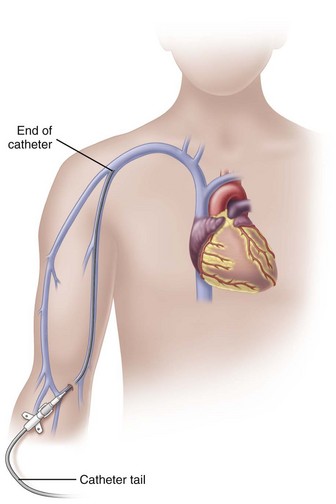
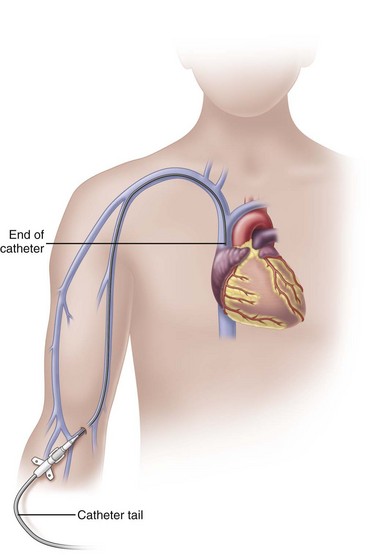
Peripherally Inserted Central Catheters
In adults, the PICC length ranges from 18 to 29 inches (45-72 cm) with the tip residing in the superior vena cava (SVC) (Fig. 15-6). Placement of the catheter tip in veins distal to the SVC is avoided. This inappropriate tip location, often called a mid-clavicular catheter, is associated with much higher rates of thrombosis than when the tip is located in the SVC. Mid-clavicular tip locations are used only when anatomic or pathophysiologic changes prohibit placing the catheter into the SVC.
PICCs are available in single-, dual-, or triple-lumen configurations and are available with both the Groshong valve and the pressure-activated safety valve (PASV). PICCs are also available as “Power PICCs” and can be used for contrast injection at a maximum of 5 mL/sec and a maximum pressure of 300 psi. They can also be connected to transducers and used to monitor central venous pressure (Bard Access Systems, 2011).
• Maximal barrier precautions upon insertion
• Chlorhexidine skin antisepsis
• Optimal catheter site selection and post-placement care with avoidance of the femoral vein for central venous access in adult patients
• Daily review of line necessity with prompt removal of unnecessary lines
Action Alert
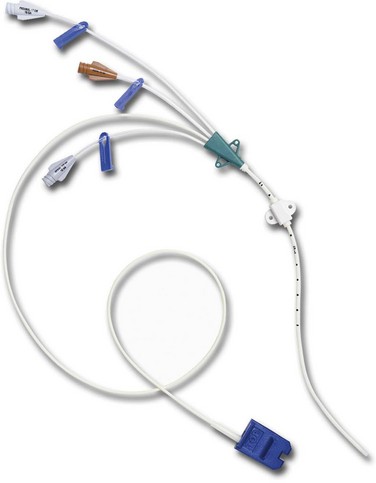
Nontunneled Percutaneous Central Venous Catheters
CVCs are usually 7 to 10 inches (18 to 25 cm) long and have one to as many as five lumens (Fig. 15-7). These catheters are also available with antimicrobial coatings such as chlorhexidine and silver sulfadiazine. The tip resides in the superior vena cava (SVC) and is confirmed by a chest x-ray. Nontunneled percutaneous CVCs are most commonly used for emergent or trauma situations, critical care, and surgery. There is no recommendation for optimal dwell time. However, these catheters are commonly used for short-term situations and are not the catheter of choice for home care or ambulatory clinic settings.
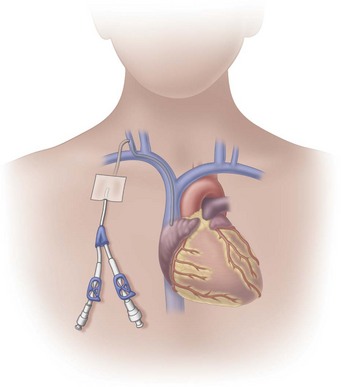
Tunneled Central Venous Catheters
Tunneled central venous catheters are VADs that have a portion of the catheter lying in a subcutaneous tunnel, separating the points where the catheter enters the vein from where it exits the skin. This separation is intended to prevent the organisms on the skin from reaching the bloodstream (Fig. 15-8). The catheter has a cuff made of a rough material that is positioned inside the subcutaneous tunnel. These cuffs commonly contain antibiotics, which also reduce the risk for infection. The tissue granulates into the cuff, providing a mechanical barrier to microorganisms and anchoring the catheter in place.
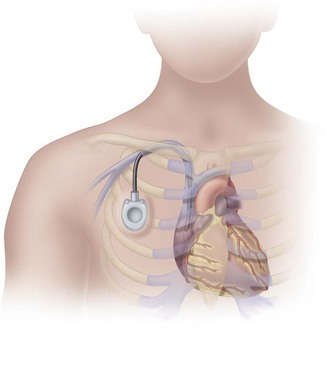
Implanted Ports
Implanted ports are very different from other central vascular access devices (CVADs). They consist of a portal body, a dense septum over a reservoir, and a catheter. They can be single- or double-lumen and come in various sizes. A subcutaneous pocket is surgically created to house the port body. The catheter is inserted into the vein and attached to the portal body. The septum is made of self-sealing silicone and is located in the center of the port body over the reservoir; the catheter extends from the side of the port body. The incision is closed, and no part of the catheter is visible externally; therefore this device has the least impact on body image (Fig. 15-9).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree