M. Linda Workman
Inflammation and the Immune Response
Learning Outcomes
Physiological Integrity

http://evolve.elsevier.com/Iggy/
Animation: Function of B Cells
Animation: Function of T Cytotoxic Cells
Animation: Inflammatory Response
Audio Glossary
Concept Map Creator
Key Points
Review Questions for the NCLEX® Examination
Inflammatory responses and the immune system work with other defenses to provide protection from harmful microorganisms and cells. As indicated in the section opener defining the concept of protection, the cells and cell products of inflammation and immunity are represented mostly by the knights and soldiers behind the castle walls. The products made by cells, such as cytokines, growth factors, and antibodies, are the weapons of the knights and soldiers. In addition, some of these cells serve as watchers and alarm systems actually within the castle walls (skin and mucous membranes). They also help maintain and repair these walls when damage occurs.
Infectious diseases are common. Most people, however, are healthy more often than they are ill. Inflammation and immunity are the major defenses that protect against disease when the body is invaded by organisms. These same defenses also help the body recover after tissue damage. For these reasons, inflammation and immunity are critical to maintaining health and preventing disease. When all the different parts and functions of inflammation and immunity are working well, the person is immunocompetent and has maximum protection against infection.
Immune function is reduced by many diseases, injuries, and medical therapies. Reduction of immune function may be temporary or permanent, but it always endangers the patient’s health. Chapter 21 discusses issues related to reduced protection from inadequate immunity or inflammatory responses. Other problems occur when immunity overfunctions or functions at inappropriate times, at which time this normally protective response becomes an enemy (Braun, 2010; Stevens, 2009). Chapters 20 and 22 discuss issues related to damage from excess or inappropriate inflammatory or immune responses.
Overview
Immunity is composed of many cell functions that protect people against the effects of injury or invasion. People interact with many other living organisms in the environment. The size of these organisms varies from large (other humans and animals) to microscopic (bacteria, viruses, molds, spores, pollens, protozoa, and cells from other people or animals). As long as organisms do not enter the body, they pose no health threat. The body has some defenses to prevent organisms from entering, such as intact skin and mucous membranes, skin surface normal flora, and natural chemicals that inhibit bacterial growth. These defenses are not perfect, and invasion of the body by organisms occurs often. Invasion occurs much more often than does an actual disease or illness because of proper immune functioning.
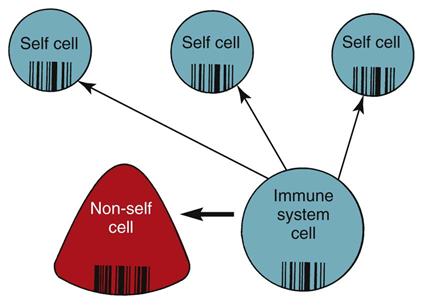
The purpose of inflammation and immunity is to provide protection by neutralizing, eliminating, or destroying organisms that invade the internal environment. To protect without harming the body, immune system cells use protective actions only against non-self proteins and cells. Immune system cells can distinguish between the body’s own healthy self cells and other, non-self proteins and cells.
Self Versus Non-Self
Non-self proteins and cells include infected body cells, cancer cells, cells from other people, and invading organisms. Recognizing self versus non-self, which is necessary to prevent healthy body cells from being destroyed along with the invaders, is called self-tolerance. The immune system cells are the only cells capable of determining self from non-self. Self-tolerance is possible because of the different proteins present on cell membranes.
Each cell is surrounded by a plasma membrane with different proteins protruding through the surface (Fig. 19-1). For example, in liver cells, many different protein types are present on the cell membrane surface. The amino acid sequence of each protein type differs from that of all other protein types. Some of these proteins are found on the liver cells of all animals (including humans) that have livers, because these protein types are specific to the liver and serve as a marker for liver tissues. Other protein types are found only on the liver cells of humans, because these protein types are specific markers for human tissues. In addition, each person’s liver cells have surface proteins that are specific to that person. These unique proteins would be identical only to the proteins of an identical sibling. These unique proteins, known as human leukocyte antigens (HLAs), are found on the surface of all body cells of that person and serve as a “universal product code” or a “cellular fingerprint” for that person. One person’s HLAs are recognized as “foreign,” or non-self, by the immune system of another person. Because the cell-surface proteins are non-self to another person’s immune system, they are antigens, which are proteins capable of stimulating an immune response.
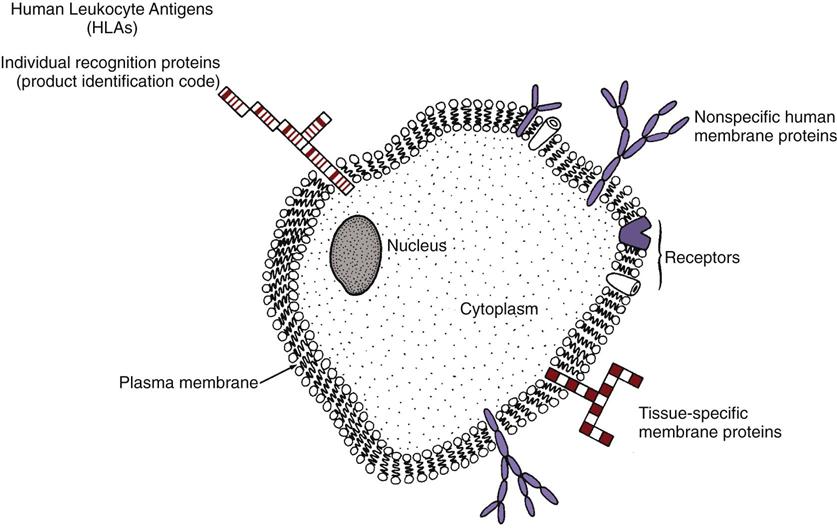
Human leukocyte antigens are on the surfaces of most body cells—not just on leukocytes. They are a normal part of the person and act as antigens only if they enter another person’s body. These antigens determine the tissue type of a person. Other names for these cellular fingerprints are human transplantation antigens, human histocompatibility antigens, and class I antigens.
Humans have about 40 major HLAs that are determined by a set of genes called the major histocompatibility complex (MHC). The specific antigens that any person has (of a large number of possible antigens) are determined by which MHC gene alleles were inherited from his or her parents (Nussbaum et al., 2007).
The HLAs are key for recognition and self-tolerance. The immune system cells constantly come into contact with other body cells and with any invader that enters the body. At each encounter, the immune system cells compare the surface protein HLAs to determine whether the encountered cell belongs in the body (Fig. 19-2). If the encountered cell’s HLAs perfectly match the HLAs of the immune system cell, the encountered cell is “self” and is not attacked by the immune system cell. If the encountered cell’s HLAs do not perfectly match the HLAs of the immune system cell, the encountered cell is non-self, or foreign. The immune system cell then takes action to neutralize, destroy, or eliminate this foreign invader.
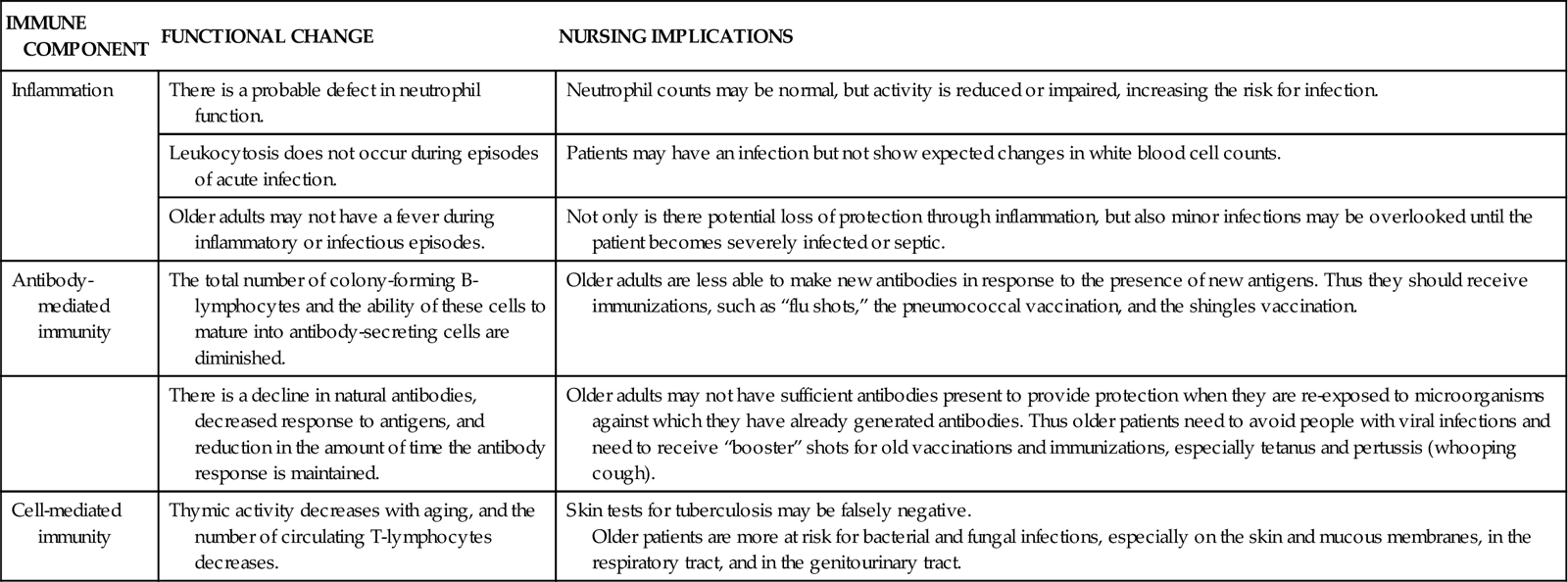
Immune function changes during a person’s life, according to nutritional status, environmental conditions, drugs, disease, and age. Immune function is most efficient when people are in their 20s and 30s and slowly declines with increasing age (Graham et al., 2006; Touhy & Jett, 2010). Older adults have decreased immune function, increasing their risk for many health problems (Chart 19-1).
Organization of the Immune System
The immune system is not located in any one body area but, rather, is influenced by many systems, especially the nervous system, the endocrine system, and the GI system. Most immune system cells come from the bone marrow. Some cells mature in the bone marrow; others leave the bone marrow and mature in different body sites. When mature, many immune system cells are released into the blood, where they circulate to most body areas and have specific effects.
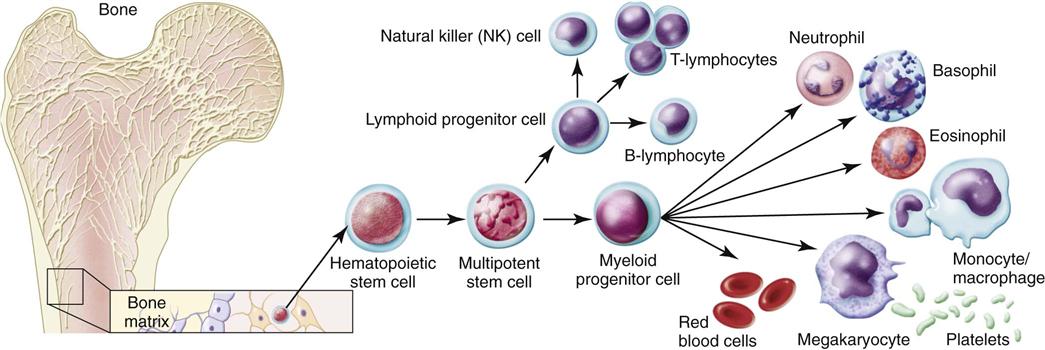
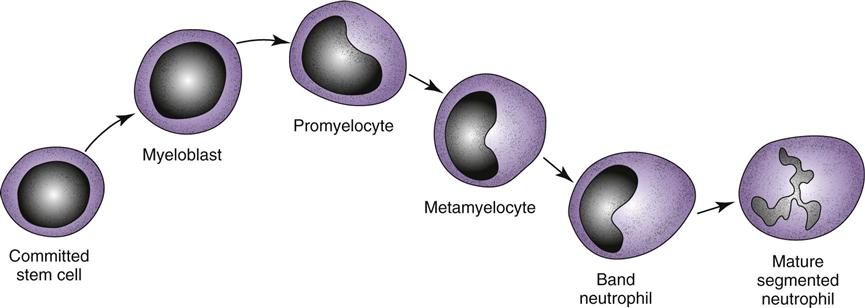
The bone marrow is the source of all blood cells, including immune system cells. The bone marrow produces immature, undifferentiated cells called stem cells (Abbas et al., 2010, Kaufman, 2011). Stem cells are pluripotent, meaning that each cell has more than one potential outcome. When the stem cell is first created in the bone marrow, it is undifferentiated. The cell is not yet committed to maturing into a specific blood cell type. At this stage, the stem cell is flexible (pluripotent) and could become any one of many mature blood cells. Fig. 19-3 shows the possible outcomes for maturation of stem cells. The type of mature cell that the stem cell becomes depends on which pathway it follows.
The maturational pathway of any stem cell depends on body needs and on the presence of specific growth factors that direct the cell to a pathway. For example, erythropoietin is a growth factor for red blood cells (RBCs). When immature stem cells are exposed to erythropoietin, they commit to the erythrocyte pathway and eventually become mature RBCs.
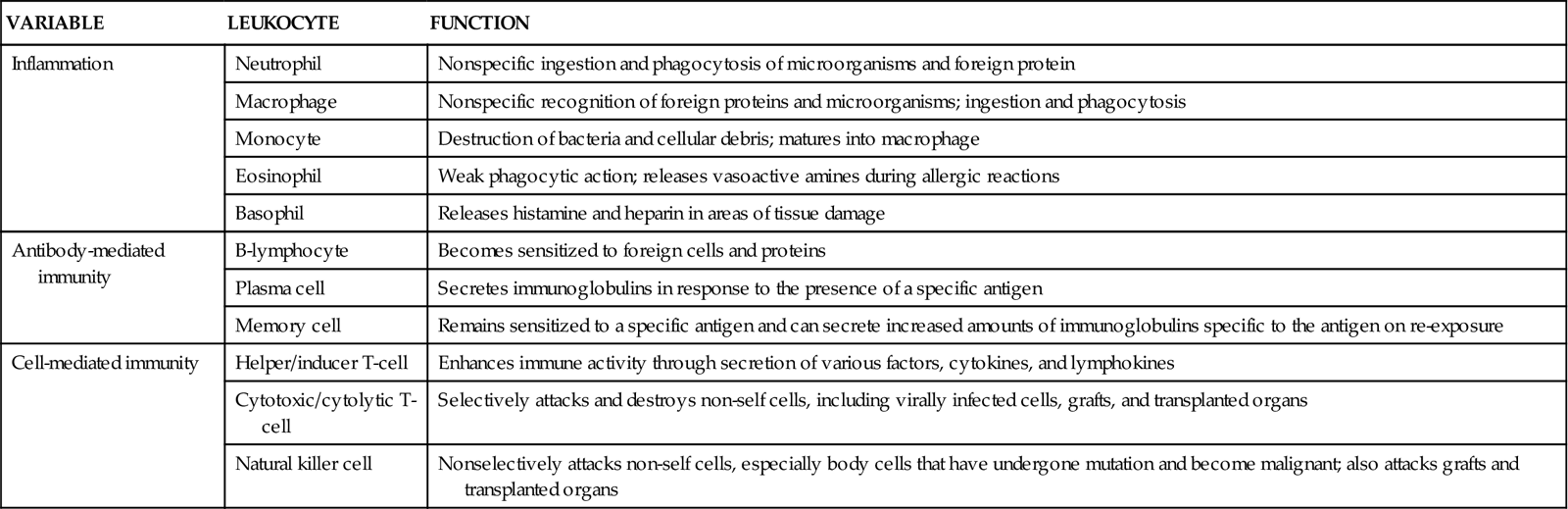
White blood cells (leukocytes or WBCs) protect the body from the effects of invasion by organisms. These cells are the immune system cells—the knight and soldiers protecting the castle inhabitants after invaders get through the castle wall. Table 19-1 lists the functions of different immune system cells. The leukocytes provide protection through many defensive actions (Abbas et al., 2010; Stevens, 2009). These actions include:
• Recognition of self versus non-self
• Destruction of foreign invaders, cellular debris, and unhealthy or abnormal self cells
• Production of antibodies directed against invaders
TABLE 19-1
IMMUNE FUNCTIONS OF SPECIFIC LEUKOCYTES
| VARIABLE | LEUKOCYTE | FUNCTION |
| Inflammation | Neutrophil | Nonspecific ingestion and phagocytosis of microorganisms and foreign protein |
| Macrophage | Nonspecific recognition of foreign proteins and microorganisms; ingestion and phagocytosis | |
| Monocyte | Destruction of bacteria and cellular debris; matures into macrophage | |
| Eosinophil | Weak phagocytic action; releases vasoactive amines during allergic reactions | |
| Basophil | Releases histamine and heparin in areas of tissue damage | |
| Antibody-mediated immunity | B-lymphocyte | Becomes sensitized to foreign cells and proteins |
| Plasma cell | Secretes immunoglobulins in response to the presence of a specific antigen | |
| Memory cell | Remains sensitized to a specific antigen and can secrete increased amounts of immunoglobulins specific to the antigen on re-exposure | |
| Cell-mediated immunity | Helper/inducer T-cell | Enhances immune activity through secretion of various factors, cytokines, and lymphokines |
| Cytotoxic/cytolytic T-cell | Selectively attacks and destroys non-self cells, including virally infected cells, grafts, and transplanted organs | |
| Natural killer cell | Nonselectively attacks non-self cells, especially body cells that have undergone mutation and become malignant; also attacks grafts and transplanted organs |
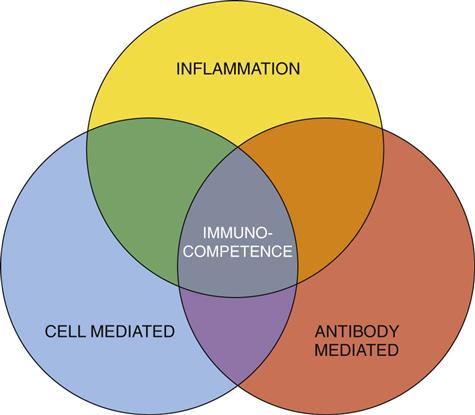
The three processes needed for human protection through immunity are (1) inflammation, (2) antibody-mediated immunity (AMI), and (3) cell-mediated immunity (CMI). Each process uses different defensive actions, and each influences or requires assistance from the other two processes (Fig. 19-4). Therefore full immunity (immunocompetence) requires the function and interaction of all three processes.
Inflammation
Inflammation, also called innate-native immunity or natural immunity, provides immediate protection against the effects of tissue injury and invading foreign proteins. Innate-native immunity is any natural protective feature of a person. It can be a barrier to prevent organisms from entering the body or can be an attacking force that eliminates organisms that have already entered the body. This type of immunity cannot be developed or transferred from one person to another and is not an adaptive response to exposure or invasion by foreign proteins.
The inflammatory responses are part of innate immunity. Other parts of innate immunity include skin, mucosa, antimicrobial chemicals on the skin, complement, and natural killer cells (Abbas et al., 2010; Stevens, 2009).
The ability to produce an inflammatory response is critical to health and well-being. Inflammation differs from AMI and CMI in two important ways:
So, inflammation triggered by a scald burn to the hand is the same as inflammation triggered by bacteria in the middle ear. How widespread the symptoms of inflammation are depends on the intensity, severity, and duration of exposure to the initiating event. For example, a splinter in the finger triggers inflammation only at the splinter site, whereas a burn injuring 50% of the skin leads to an inflammatory response involving the entire body.
Inflammatory responses start tissue actions that cause visible and uncomfortable symptoms. Despite the discomfort, these actions are important in ridding the body of harmful organisms. However, if the inflammatory response is excessive, tissue damage may result (Landis, 2009). Inflammatory responses also help start both antibody-mediated and cell-mediated actions to activate a full immune response.
Infection
A confusing issue about inflammation is that this process occurs in response to tissue injury, as well as to invasion by organisms. Infection is usually accompanied by inflammation; however, inflammation can occur without infection. Examples of inflammation without infection include joint sprain injuries, myocardial infarction, and blister formation. Examples of inflammation caused by noninfectious invasion include allergic rhinitis, contact dermatitis, and other allergic reactions. Inflammations from infection include otitis media, appendicitis, and viral hepatitis, among many others. Inflammation does not always mean that an infection is present.
Cell Types Involved in Inflammation
The leukocytes involved in inflammation are neutrophils, macrophages, eosinophils, and basophils. Neutrophils and macrophages use phagocytosis to destroy and eliminate foreign invaders. Basophils and eosinophils release chemicals that act on blood vessels to cause tissue-level responses that help neutrophil and macrophage actions.
Neutrophils
Mature neutrophils make up between 55% and 70% of the normal total white blood cell (WBC) count. Neutrophils come from the stem cells and complete the maturation process in the bone marrow (Fig. 19-5). They are also called granulocytes because of the large number of granules present inside each cell. Other names for neutrophils are based on their appearance and maturity. Mature neutrophils are also called segmented neutrophils (“segs”) or polymorphonuclear cells (“polys,” PMNs) because of their segmented nucleus. Less mature neutrophils are called band neutrophils (“bands” or “stabs”) because of their nuclear shape.
Usually, growth of a stem cell into a mature neutrophil requires 12 to 14 days. This time is shortened by the presence of specific growth factors (cytokines), such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF). The purpose and action of cytokines are described on p. 314 in the Cytokines section.
In the immunocompetent healthy person, more than 100 billion fresh, mature neutrophils are released from the bone marrow into the circulation daily (Abbas et al., 2010; Stevens, 2009). This huge production is needed because the life span of each neutrophil is short—about 12 to 18 hours.
Neutrophil function provides protection after invaders, especially bacteria, enter the body. Although the neutrophils are the largest group of circulating leukocytes, each cell is small. This powerful army destroys invaders by phagocytosis and enzymatic digestion, although each cell is small and can take part in only one episode of phagocytosis.
Mature neutrophils are the only stage of this cell capable of phagocytosis. Because this cell type is responsible for continuous, instant, nonspecific protection against organisms, the percentage and actual number of mature circulating neutrophils are used to measure a patient’s risk for infection: the higher the numbers, the greater the resistance to infection. This measurement is the absolute neutrophil count (ANC), also called the absolute granulocyte count or total granulocyte count.
The differential of a normal white blood cell count shows the number and percent of the different types of circulating leukocytes (Table 19-2). Usually, most neutrophils released into the blood from the bone marrow are segmented neutrophils; only a small percentage are band neutrophils. Less mature neutrophil forms should not be in the blood. Some problems, such as sepsis, cause the neutrophils in the blood to change from being mostly segmented neutrophils to being less mature forms. This situation is termed a left shift or bandemia because the segmented neutrophil, which is seen at the far right of the neutrophil pathway (see Fig. 19-5), is no longer the most numerous type of circulating neutrophils. Instead, more of the circulating cells are bands—the less mature cell type found farther left on the neutrophil pathway.
TABLE 19-2
VALUES OF A WHITE BLOOD CELL DIFFERENTIAL FOR PERIPHERAL BLOOD REPRESENTING A NORMAL COUNT
| WBC TYPE | % | /MM3 |
| TOTAL WBC | 100 | 10,000 |
| Segs | 62 | 6200 |
| Bands | 5 | 500 |
| Monos | 3 | 300 |
| Lymphs | 28 | 2800 |
| Eosins | 1.5 | 150 |
| Basos | 0.5 | 50 |
A left shift indicates that the patient’s bone marrow cannot produce enough mature neutrophils to keep pace with the continuing infection and is releasing immature neutrophils into the blood. Unfortunately, most of these immature cells are of no benefit because they are not capable of phagocytosis.
Macrophages
Macrophages come from the committed myeloid stem cells in the bone marrow and form the mononuclear-phagocyte system. The stem cells first form monocytes, which are released into the blood at this stage. Until they mature, monocytes have limited activity. Most monocytes move from the blood into body tissues, where they mature into macrophages. Some macrophages become “fixed” in position within the tissues, whereas others can move within and between tissues. Macrophages in various tissues have slightly different appearances and names (Table 19-3). The liver, spleen, and intestinal tract contain large numbers of these cells.
TABLE 19-3
| TISSUE | MACROPHAGE |
| Lung | Alveolar macrophage |
| Connective tissue | Histiocyte |
| Brain | Microglial cell |
| Liver | Kupffer cell |
| Peritoneum | Peritoneal macrophage |
| Bone | Osteoclast |
| Joint | Synovial type A cell |
| Kidney | Mesangial cell |
Macrophage function provides protection in several ways. These cells are important in immediate inflammatory responses and also stimulate the longer-lasting immune responses of antibody-mediated immunity (AMI) and cell-mediated immunity (CMI). Specific macrophage functions include phagocytosis, repair, antigen presenting/processing, and secretion of cytokines that help control the immune system.
The inflammatory function of macrophages is phagocytosis. Macrophages can easily distinguish between self and non-self, and their large size makes them very effective at trapping invading cells. Unlike neutrophils, macrophages have long life spans and can take part in many phagocytic events.
Basophils
Basophils come from myeloid stem cells and make up only about 1% of the total circulating WBC count. These cells cause the manifestations of inflammation.
Basophil function results in actions on blood vessels through the activity of basophil chemicals (vasoactive amines). These chemicals include heparin, histamine, serotonin, kinins, and leukotrienes. When released into the blood, most of these chemicals act on smooth muscle and blood vessel walls. Heparin inhibits blood and protein clotting. Histamine constricts small veins, inhibiting blood flow and decreasing venous return. This effect causes blood to collect in capillaries and arterioles. Kinins dilate arterioles and increase capillary permeability. These actions cause blood plasma to leak into the interstitial space (vascular leak syndrome).
Eosinophils
Eosinophils come from the myeloid line and contain many vasoactive chemicals. Usually, only 1% to 2% of the total white blood cell (WBC) count is composed of eosinophils.
Eosinophil function is very active against infestations of parasitic larvae and also limits inflammatory reactions. The eosinophil granules contain many different substances. Some are enzymes that degrade the vasoactive chemicals released by other leukocytes. This is why the number of circulating eosinophils increases during an allergic response.
Phagocytosis
A key process of inflammation is phagocytosis, the engulfing and destruction of invaders. This action also rids the body of debris after tissue injury. Neutrophils and macrophages are most efficient at phagocytosis. Phagocytosis involves the seven steps shown in Fig. 19-6.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree