On completion of this chapter the reader will be able to: • Identify the principal cardiorespiratory changes that occur during the transition to extrauterine life. • Identify the immature physiologic functioning of each body system and its significance to nursing care of the newborn. • Perform an initial and transitional assessment of a newborn, including the Apgar score and gestational age assessment. • Perform a newborn physical assessment based on recognition of expected normal findings. • Outline a nursing plan of care for a well newborn. http://evolve.elsevier.com/wong/essentials In the alveoli, the surface tension of the fluid is reduced by surfactant, a substance produced by the alveolar epithelium that coats the alveolar surface. The effect of surfactant in facilitating breathing is discussed in relation to respiratory distress syndrome (see Chapter 9). The most important factors controlling ductal closure are the increased oxygen concentration of the blood and the fall in endogenous prostaglandins. The foramen ovale closes functionally at or soon after birth. The ductus arteriosus is closed functionally by the fourth day. Anatomic closure takes considerably longer. Failure of the ductus arteriosus or foramen ovale to close results in persistence of fetal shunting of blood away from the lungs (see Chapter 25). • The newborn’s large surface area facilitates heat loss to the environment, although this is partially compensated for by the newborn’s usual position of flexion, which decreases the amount of surface area exposed to the environment. • The newborn’s thin layer of subcutaneous fat provides poor insulation for conservation of heat. • The newborn’s mechanism for producing heat is different from that of the adult, who can increase heat production through shivering. A chilled neonate cannot shiver but produces heat through nonshivering thermogenesis (NST), which involves increased metabolism and oxygen consumption. The principal thermogenic sources are the heart, liver, and brain. An additional source, once believed to be unique to newborns (Zingaretti, Crosta, Vitali, and others, 2009), is known as brown adipose tissue, or brown fat. Brown fat, which owes its name to its larger content of mitochondrial cytochromes, has a greater capacity for heat production through intensified metabolic activity than does ordinary adipose tissue. Heat generated in brown fat is distributed to other parts of the body by the blood, which is warmed as it flows through the layers of this tissue. Superficial deposits of brown fat are located between the scapulae, around the neck, in the axillae, and behind the sternum. Deeper layers surround the kidneys, trachea, esophagus, some major arteries, and adrenals. The location of brown fat may explain why the nape of the neck often feels warmer than the rest of the infant’s body. The blood volume of the newborn depends on the amount of placental transfer of blood. The blood volume of a full-term infant is about 80 to 85 ml/kg of body weight. Immediately after birth, the total blood volume averages 300 ml, but depending on how long umbilical cord clamping is delayed or if the umbilical cord is milked, as much as 100 ml can be added to the blood volume (Rabe, Jewison, Alvarez, and others, 2011). Blood values for the newborn are listed in Appendix C. Some salivary glands are functioning at birth, but the majority do not begin to secrete saliva until about age 2 to 3 months, when drooling is frequent. Stomach capacity varies in the first few days of life, from about 5 ml on day 1 to about 60 ml on day 3 (Spangler, Randenberg, Brenner, and others, 2008); thus, infants require frequent small feedings. The colon also has a small volume; newborns may have a bowel movement after each feeding. Newborns who breastfeed usually have more frequent feedings and more frequent stools than infants who receive formula. An infant’s intestine is longer in relation to body size than that of the adult. Therefore, there are a larger number of secretory glands and a larger surface area for absorption compared with an adult’s intestine. Infants have rapid peristaltic waves and simultaneous nonperistaltic waves along the entire esophagus, which propel nutrients forward. The relative immaturity of the peristaltic waves combined with decreased lower esophageal sphincter (LES) pressure, inappropriate relaxation of the LES, and delayed gastric emptying make regurgitation a common occurrence. Progressive changes in the stooling pattern indicate a properly functioning gastrointestinal tract (Box 8-1). The neonatal gastrointestinal mucosa may perform an important function as a barrier to foreign antigens. Both immune and nonimmune factors may play a vital role in decreasing the absorption of antigens capable of causing serious neonatal illness; however, the functional capacity of this system may be immature or altered. Feeding an infant human milk increases the effectiveness of this defense mechanism (Le Huërou-Luron, Blat, and Boudry, 2010). 1. The initial assessment, which includes the Apgar scoring system 2. Transitional assessment during the periods of reactivity 3. Assessment of gestational age The most frequently used method to assess newborns’ immediate adjustment to extrauterine life is the Apgar scoring system, which is based on newborn heart rate, respiratory effort, muscle tone, reflex irritability, and color (Table 8-1). Each item is given a score of 0, 1, or 2. Evaluations of all five categories are made at 1 and 5 minutes after birth and repeated until the infant’s condition stabilizes. Total scores of 0 to 3 represent severe distress, scores of 4 to 6 signify moderate difficulty, and scores of 7 to 10 indicate absence of difficulty in adjusting to extrauterine life. The Apgar score is affected by the degree of physiologic immaturity, infection, congenital malformations, maternal sedation or analgesia, and neuromuscular disorders. TABLE 8-1 INFANT EVALUATION AT BIRTH—APGAR SCORING SYSTEM The Apgar score reflects the general condition of the infant at 1 and 5 minutes based on the five parameters described previously. The Apgar score is not a tool, however, that stands on its own to interpret past events, determine need for newborn resuscitation, or predict future events linked to the infant’s eventual neurologic or physical status. Considerable discussion and controversy have centered on Apgar scoring because of its misuse as an indicator for the presence or absence of perinatal asphyxia in the medicolegal field (American Academy of Pediatrics [AAP] Committee on Fetus and Newborn, and American College of Obstetricians and Gynecologists [ACOG], Committee on Obstetric Practice, 2006, reaffirmed 2008). Assessment of gestational age is an important criterion because perinatal morbidity and mortality are related to gestational age and birth weight. A frequently used method of determining gestational age is the New Ballard Scale (NBS) by Ballard, Khoury, Wedig, and others (1991) (Fig. 8-1, A). This scale, an abbreviated version of the Dubowitz scale, assesses six external physical and six neuromuscular signs. Each sign has a number score, and the cumulative score correlates with a maturity rating of 20 to 44 weeks of gestation. The NBS includes –1 and –2 scores that reflect signs of extremely preterm infants, such as fused eyelids; imperceptible breast tissue; sticky, friable, transparent skin; no lanugo; and square-window (flexion of wrist) angle of greater than 90 degrees (see Fig. 8-1, A, and the description of the tests in Box 8-2). For infants with a gestational age of at least 26 weeks, the examination may be performed up to 96 hours after birth; however, it is recommended that the initial examination be performed within the first 48 hours of life. In a study of preterm infants ranging from 29 to 35 weeks at birth, Ballard scores completed after 7 days after birth were found to either overestimate or underestimate gestational age by up to 2 weeks (Sasidharan, Dutta, and Narang, 2009). In a blinded Spanish study, Marín, Martín, Lliteras, and others (2006) compared estimations of gestational age using NBS versus ultrasonography or the mother’s last menstrual period. Researchers found general agreement between NBS and ultrasonography or last menstrual period; however, they noted that NBS tends to overestimate gestational age in very preterm newborns and in infants whose mothers had received prenatal corticosteroid therapy. Intrauterine growth curves are used to classify infants according to birth weight and gestational age. The primary intrauterine growth charts that provide national reference data include the work of Alexander, Himes, Kaufman, and others (1996), which is representative of more than 3.1 million live births in the United States, and Thomas, Peabody, Turnier, and others (2000). Olsen, Groveman, Lawson, and others (2010) published new intrauterine growth curves based on more than 257,000 infants in the United States, noting that use of a contemporary, large, and racially diverse U.S. sample has produced intrauterine growth curves that differ from those produced earlier. Thomas, Peabody, Turnier, and others (2000) concluded that intrauterine growth measured by head circumference, birth weight, and length varies according to race and gender. These researchers also found that altitude did not seem to significantly affect birth weight, as has been suggested by other authors. It is recommended that readers access and use the most current intrauterine growth chart specific to the referent population being evaluated. Classification of infants at birth by both birth weight and gestational age provides a more satisfactory method for predicting mortality risks and providing guidelines for management of the neonate than estimating gestational age or birth weight alone. The infant’s birth weight, length, and head circumference are plotted on standardized graphs that identify normal values for gestational age (for birth weight see Fig. 8-1, B). Infants whose weight is appropriate for gestational age (AGA) (between the 10th and 90th percentiles) can be presumed to have grown at a normal rate regardless of the time of birth—preterm, term, or postterm. Infants who are large for gestational age (LGA) (above the 90th percentile) can be presumed to have grown at an accelerated rate during fetal life; small-for-gestational-age (SGA) infants (below the 10th percentile) can be assumed to have intrauterine growth restriction or delay. When gestational age is determined according to a standardized gestational age scale such as the NBS, the newborn will fall into one of the following nine possible categories for birth weight and gestational age: AGA—term, preterm, postterm; SGA—term, preterm, postterm; LGA—term, preterm, postterm. Figure 8-2 illustrates the disparity between birth weights of three preterm infants of the same gestational age, 32 weeks. Birth weight and gestational age both influence morbidity and mortality; the lower the birth weight and gestational age, the higher the morbidity and mortality. Head-to-heel length is also measured. Because of the usual flexed position of infants, it is important to extend the legs completely when measuring total body length. The average length of newborns is 48 to 53 cm (19–21 inches) (Fig. 8-3). Foote and colleagues (2011) have developed an evidence-based practice guideline for measuring length in infants and children. Another category of measurements is vital signs. Axillary temperatures are taken because insertion of a thermometer into the rectum can potentially cause perforation of the mucosa if performed incorrectly (see Table 6-3 and Fig. 8-4). Core body temperature varies according to the periods of reactivity but is usually 36.5° to 37.6° C (97.7°–99.7° F). Skin temperature is slightly lower than core body temperature. Therefore axillary temperature is generally less than rectal temperature, measuring about 0.2° C lower (Hussink, van Berkel, and de Beaufort, 2008). Because brown adipose tissue is located in the axillary pocket, axillary readings may be elevated whenever NST occurs. However, axillary readings may be normal in cold-stressed infants when NST is not triggered or is overwhelmed.
Health Promotion of the Newborn and Family
Adjustment to Extrauterine Life
Immediate Adjustments
Respiratory System
Circulatory System
![]() As important as the initiation of respiration are the circulatory changes that allow blood to flow through the lungs. These changes, which occur more gradually, are the result of pressure changes in the lungs, heart, and major vessels. The transition from fetal to postnatal circulation involves the functional closure of the fetal shunts: the foramen ovale, the ductus arteriosus, and eventually the ductus venosus. (For a review of fetal circulation, see Chapter 25.) Increased blood flow dilates the pulmonary vessels, pulmonary vascular resistance decreases, and systemic resistance increases, thus maintaining blood pressure (BP). As the pulmonary vessels receive blood, the pressure in the right atrium, right ventricle, and pulmonary arteries decreases. Left atrial pressure increases above right atrial pressure, with subsequent foramen ovale closure. With the increase in pulmonary blood flow and dramatic reduction of pulmonary vascular resistance, the ductus arteriosus begins to close.
As important as the initiation of respiration are the circulatory changes that allow blood to flow through the lungs. These changes, which occur more gradually, are the result of pressure changes in the lungs, heart, and major vessels. The transition from fetal to postnatal circulation involves the functional closure of the fetal shunts: the foramen ovale, the ductus arteriosus, and eventually the ductus venosus. (For a review of fetal circulation, see Chapter 25.) Increased blood flow dilates the pulmonary vessels, pulmonary vascular resistance decreases, and systemic resistance increases, thus maintaining blood pressure (BP). As the pulmonary vessels receive blood, the pressure in the right atrium, right ventricle, and pulmonary arteries decreases. Left atrial pressure increases above right atrial pressure, with subsequent foramen ovale closure. With the increase in pulmonary blood flow and dramatic reduction of pulmonary vascular resistance, the ductus arteriosus begins to close.
Physiologic Status of Other Systems
Thermoregulation
Hematopoietic System
Gastrointestinal System
Nursing Care of the Newborn and Family
Assessment
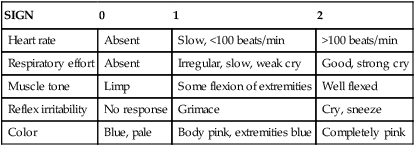
Initial Assessment: Apgar Scoring
SIGN
0
1
2
Heart rate
Absent
Slow, <100 beats/min
>100 beats/min
Respiratory effort
Absent
Irregular, slow, weak cry
Good, strong cry
Muscle tone
Limp
Some flexion of extremities
Well flexed
Reflex irritability
No response
Grimace
Cry, sneeze
Color
Blue, pale
Body pink, extremities blue
Completely pink
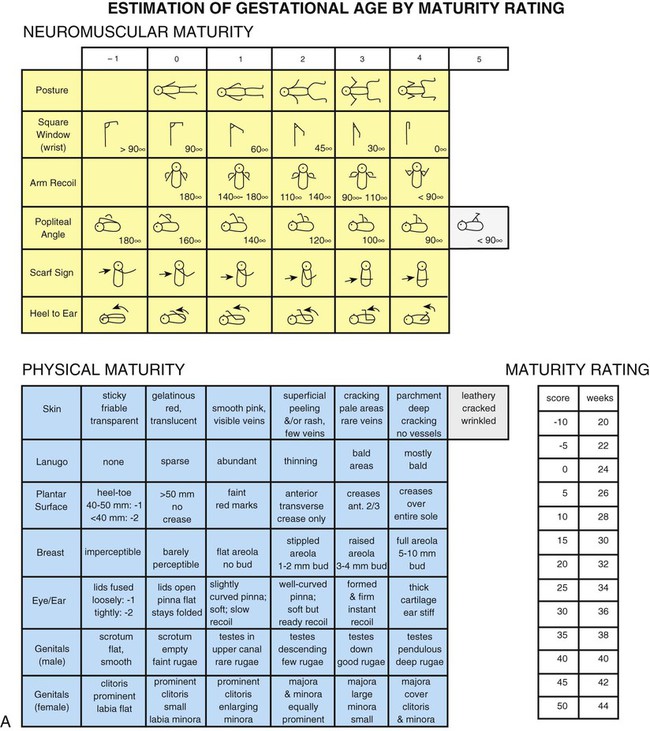
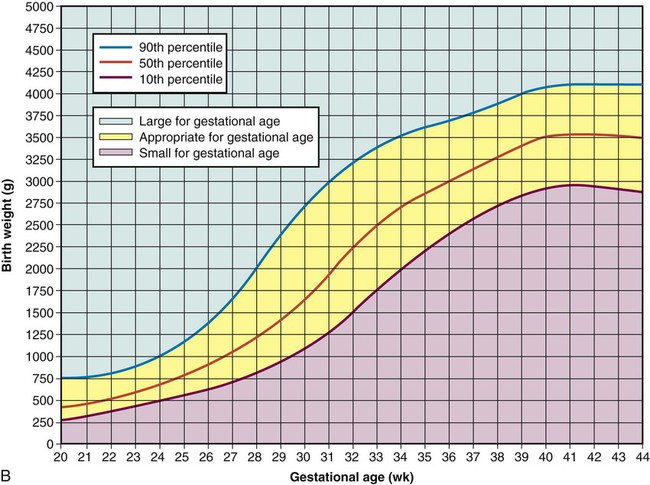
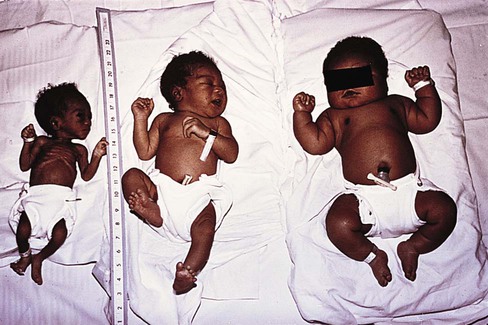
Clinical Assessment of Gestational Age
Weight Related to Gestational Age
General Measurements
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Health Promotion of the Newborn and Family
Get Clinical Tree app for offline access