Ultrafiltration and hydrostatic pressure
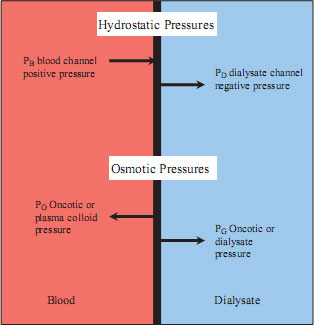
As blood is pumped through a dialyser, a positive pressure will be exerted on the membrane. The pressure in the space on the opposite side of the membrane will be lower, whether or not the space is filled with dialysate. As a result, fluid and small solutes will move from the area of greater pressure to the area of lower pressure (see Figure 8.2).
Figure 8.2 Transmembrane pressures. Overall pressure difference over the dialyser membrane = (PB+ PD) – (PO + PG). However, as the osmotic pressures are small in relation to the hydrostatic pressures the transmembrane pressure (TMP) = (PB + PD).
Ultrafiltration and convection
As a result of hydrostatic pressure, fluid will move across the semipermeable membrane, and this process is called ultrafiltration. The rate of ultrafiltration depends on the permeability of the membrane and the hydrostatic pressure exerted upon it. The sum of the positive pressure in the blood compartment and the negative pressure in the dialysate compartment equals the transmembrane pressure (TMP).
The removal of fluid by ultrafiltration also results in the removal of solute, or those molecules dissolved in the water; this process is known as convection or solvent drag. Again, the higher the permeability of the membrane, the higher the removed volume of fluid and contained solute will be.
During conventional haemodialysis treatment, fluid will be removed by ultrafiltration, but this is usually a small amount (2–3 L) and will not provide significant removal of waste products by convection. Haemodialysis has traditionally been the standard form of extracorporeal treatment: haemofiltration and haemodiafiltration are becoming more common therapies in the treatment of patients requiring on-going renal replacement therapy. Some centres, however, now routinely use intermittent haemodiafiltration in place of conventional haemodialysis. For the purpose of this chapter the reference is to standard haemodialysis unless otherwise stated.
Molecular weight and solute movement
As well as diffusion and convection there are a number of other influences on the ‘clearance’ or removal of a solute across the dialyser membrane. The smaller the molecular size of the solute the easier it will pass across the membrane. Urea, creatinine and electrolytes will dialyse easily. However, toxins with a larger molecular size, such as β2-microglobubin (β2M) will be cleared in smaller volumes. Movement across the dialyser is also dependent upon the permeability of the membrane (called the co-efficient of ultrafiltration (KUf): see below). High flux dialysers will enable greater clearance of middle-sized molecules, such as β2M, in comparison to low-flux dialysers.
Flow through the dialyser
Haemodialysis depends on diffusion for the efficient clearance of waste products. The patient’s blood is pumped through the circuit (via blood tubing) on one side of the membrane whilst a physiological dialysis fluid (dialysate) is passed through the circuit (via dialysate tubing) on the opposite side of the membrane. The faster the blood flows through the dialyser, the greater the amount of blood that is ‘processed’ in a given time. Efficiency of dialysis is also therefore dependent upon optimising the blood flow through the dialyser.
To optimise the concentration gradient, the blood and the dialysate flow in opposite directions (countercurrent flow). This maintains an optimum concentration gradient throughout the dialyser.
Dialysate flows through the dialyser at a rate of 500 ml/min. Lower flow rates are associated with lower clearances. Some units increase the dialysate flow above the standard 500 ml, using the rule of thumb of 1:2 ratio of blood flow to dialysate flow. However, there is conflicting evidence on whether this significantly improves clearance rates. Bhimani et al.(2008) found that a high dialysate flow rate increases phosphate clearance, but not urea or β2M.
Principles of Haemodiafiltration
Haemodiafiltration (HDF) continues to gain in popularity as the treatment of choice for many patients. This increase in popularity has been aided by new machine technology allowing the production of sterile replacement fluid at the point of treatment, so there is no longer the need to purchase, store and handle large bags of sterile solution during treatment. Space constraints do not allow more than a simple introduction to HDF within this chapter.
The principles of haemodialysis described above apply to HDF, but the treatment has a greater reliance on the removal of solutes, particularly middle molecules, through convection. Leypolt (2000) argues that HDF is more efficient at removing middle molecules (5–50 kDa in size). In addition, compared with conventional HD, convective therapies such as HDF and HF, reduce intradialytic symptomatic hypotension (ISH) in long-term patients on dialysis (Locatelli et al. 2010). One study (858 patients over an 18-year period) found no benefit for HDF over high-flux HD with respect to anaemia management, nutrition, mineral metabolism, and BP control (Vilar et al. 2009). However these authors recommended that the mortality benefit associated with HDF that was found in their programme requires confirmation in a large randomised, controlled trial. A more recent trial (Grooteman et al. 2012) did not detect a beneficial effect of haemodiafiltration on all-cause mortality and cardiovascular events compared with low-flux haemodialysis. Blankestijn et al. (2010) summarises the available clinical evidence on haemodiafiltration.
Diffusion remains the driving force for solute removal in HDF as it does in haemodialysis (HD) (see Figure 8.3). However, solute removal is influenced by the dialyser membrane characteristics, and whereas low-flux membranes whilst having a high diffusive permeability, are not as effective as the solute molecular size increases. Haemodiafiltration requires high flux membranes to allow the principles of convection to remove the larger molecules along with (see Figure 8.3) the ultrafiltrate.
Figure 8.3 Comparison of haemodialysis, haemofiltration and haemodiafiltration (used with kind permission from B.Braun Medical).
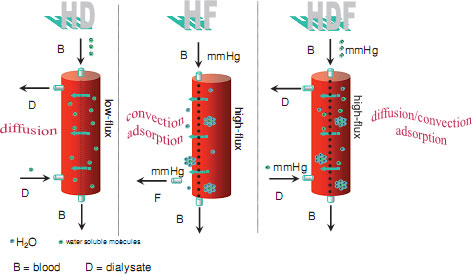
Haemofiltration (HF) relies solely on convection to remove solutes. Because of this, high ultrafiltration rates are required to achieve an effective treatment, resulting in high volumes of replacement fluid. Haemodiafiltration uses a combination of diffusion and convection. However, their effects are not additive, because the two processes interfere with each other (Lebedo and Blankestijne 2010). Diffusion reduces concentration of small molecules, leaving less for convective transport. Likewise convection reduces blood flow through the dialyser, and consequently the diffusive force. These conflicts can lead to multiple alarms if the nurse is not experienced and able to adjust the UF rate to achieve an optimal treatment.
High convective volumes have been recommended to maximise middle molecular weight (MMW) solute removal during HDF, in the DOPPS study, high efficiency HDF (arbitrarily defined as infusion volumes 15–24 L per treatment) appeared to be related to improved survival, whereas low-efficiency HDF (infusion volume <15 L per treatment) did not (Canaud et al. 2006).
There remains a debate regarding the most effective method of infusing the replacement fluid required during HDF, either predilution or postdilution. Predilution relies on the replacement fluid being infused into the blood lines before the dialyser. This can resolve problems with low ultrafiltration volumes and high TMP values associated with haemoconcentration. However, the blood is diluted when entering the dialyser, which will affect diffusion and convention. Diffusion is reduced due to the dilution effect, and convention is also reduced as the ultrafiltrate contains less solutes.
Postdilution requires infusion of the replacement fluid after the dialyser. This does allow an accurate measure of the HDF dose as the ultrafiltration is equal to the convention volume. However, it may be difficult to achieve high UF volumes due to haemoconcentration of the blood as it passes through the dialyser resulting in high TMP alarms and the possibility of interrupted treatment times.
A number of studies have shown that outcomes associated with HDF are better when compared with outcomes of patients receiving haemodialysis (Jirka et al. 2006; Canaud et al. 2006). However, these studies have mainly compared HDF against low flux dialysis. The CONTRAST study has not found a major benefit for HDF in relation to all-cause mortality and cardiovascular events when compared with low flux haemodialysis (Grootman et al. 2012). The authors have suggested that the findings may be explained by a number of factors. The loss of nutrients during HDF may outweigh the beneficial effects of higher toxin clearance. The study period of just over 3 years may be too short to show longer term benefits. Also the effect of HDF may be associated with the use of ultrapure water, which is necessary for this form of treatment. Poor water quality will have an impact on patient treatment. There is no information in any of the studies relating to the water quality of the comparator low flux haemodialysis treatments.
Access for Haemodialysis
The success of haemodialysis therapy depends almost entirely on the adequacy of the blood flow through the dialyser. Optimal clearance of waste products depends on dialysate flow rate, membrane permeability, membrane surface area, duration of dialysis and, most significantly, blood flow rate. Dysfunctional access will therefore adversely affect dialysis adequacy and consequently increase patient mortality and morbidity (KDOQI 2006).
The nurse has a responsibility to ensure that the prescribed blood flow is achieved whenever possible. Poor access should be addressed as a priority. Various types of access can be used for haemodialysis which fall broadly into two categories:
- Arteriovenous fistulae (AVF) and arteriovenous grafts.
- Percutaneous access, including jugular, subclavian and femoral lines, which can be either temporary or permanent.
For those with established renal failure the AVF is preferred, as there is evidence of long-term patency, improved flow rates and fewer complications than other methods (Fluck and Kumwenda 2011). The National Services Framework for Renal Services Part 1 (Department of Health 2004) set down a standard that patients should have vascular access created in a timely manner prior to renal replacement therapy being required. Planning for creation of vascular should be undertaken when the patient is in Stage 4 CKD (Fluck and Kumwenda 2011). However, the creation and maintenance of long-term access still remains one of the most challenging aspects of caring for those with renal disease, particularly patients with vascular disease, older people and those with diabetes mellitus (Konner 2004; Ernandez et al. 2005; Vasquez 2009).
There have been a number of initiatives around the world to decrease the reliance on central venous catheters as vascular access for haemodialysis. The Fistula First Campaign has been running in the United States for over a decade with varying success across different regions. In the United Kingdom the Department of Health has included a quality measure within the Renal Tariff to encourage the use of native fistulae as the vascular access of choice. The Renal Association (Fluck and Kumwenda 2011) is championing a quality marker of a minimum of 65% of patients with established renal failure who are on haemodialysis to have a fistula or graft.
The arteriovenous fistula
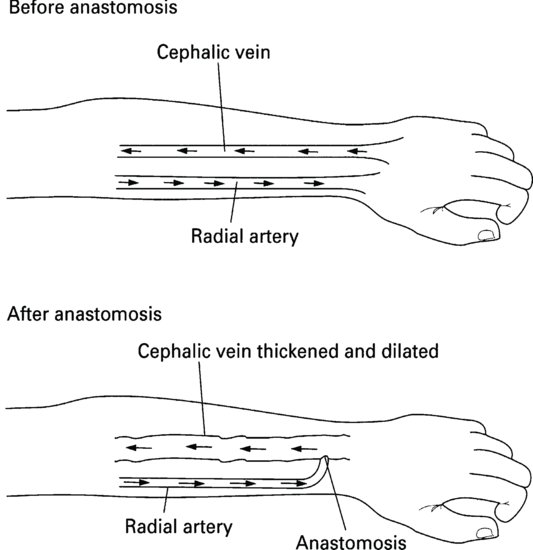
The AVF is created during a surgical procedure to anastomose an artery and a vein (Figure 8.4). Most commonly the radial artery and the cephalic vein are used in the patient’s nondominant forearm. Other sites include the upper arm: the brachial artery and cephalic vein or brachial artery and basilic vein.
As a result of the anastomosis, blood from the artery is forced into the vein where it flows in a retrograde direction. The increased blood flow and pressure cause the vein to thicken and dilate. Once established, blood flow through the fistula of up to 800–1000 ml/min can be achieved.
Ideally the patient should have the fistula created at least 3–4 months before the need for dialysis arises. This will ensure that the operation is performed when the patient is well and will allow the fistula time to mature, preventing the need for insertion of temporary access and the associated risk of infection. Many renal centres have a policy that medical and nursing staff should avoid phlebotomy or cannulation into the forearms of patients with suspected renal disease. This precaution will help to prevent damage to vessels that may subsequently be required for fistula formation. Repeated use of subclavian central venous catheters prior to fistula formation can also cause swollen arms and dilated chest veins following fistulae formation, so should be avoided (Naroienejad et al. 2010).
Formation of the Arteriovenous Fistula
Preoperative care
Patients should be given every opportunity to participate in their plan of care and all aspects of treatment should be discussed with them. As part of the patient’s predialysis preparation and education a full explanation of the surgical procedure and aftercare should be given to the patient. The patient may wish to visit and speak with someone who has a well-established functioning fistula to find out what it looks and feels like.
Information should include explanation that the fistula will be cannulated each dialysis, often using a different site (see explanation of the rope ladder puncture, below). Also it is helpful if the needles that will routinely be used should be available for the patient to see and examine. Patients should be well hydrated before surgery, and above their target weight if recently dialysed. This reduces the chance of post-operative hypotension which would adversely affect the blood-flow through the fistula, increasing the chance of thrombosis.
Postoperative care
In addition to routine postoperative care the nurse should ensure that the following specific postoperative care is carried out:
- The limb should be kept warm and well supported to help peripheral circulation.
- Blood pressure should be monitored closely and maintained at a minimum of 100 mmHg systolic. If the blood pressure falls below this, peripheral blood flow may be affected, with an increased risk of fistula thrombosis. It may be advisable to avoid antihypertensive therapy in the postoperative period.
- The wound site should be examined regularly for signs of excess bleeding or swelling.
- The blood flow through the fistula should be checked regularly by completing the following observations:
- Placing a stethoscope lightly over the incision, a ‘whooshing’ sound should be heard. This is called a ‘bruit’. The bruit should be loudest near the incision and it gradually becomes softer as the stethoscope is moved further up the vessel.
- Placing a hand lightly over the incision site, a buzzing sensation should be felt. This is called the ‘thrill’.
- Placing a stethoscope lightly over the incision, a ‘whooshing’ sound should be heard. This is called a ‘bruit’. The bruit should be loudest near the incision and it gradually becomes softer as the stethoscope is moved further up the vessel.
- The bruit and thrill should be checked regularly (half-hourly at first) and the patients should be taught how to perform these observations as soon as they are able.
- Before discharge, the patient should be informed how to care for the fistula and advised to avoid using the fistula arm for carrying heavy loads and to avoid tight or restrictive clothing on the arm. Hand exercises (such as clenching and releasing pressure on a squash ball or small bandage) may promote fistula maturation. The patient must also be advised to inform nonrenal doctors and nurses that the arm should never be used for phlebotomy, cannulation or for recording blood pressure, as all of these may result in permanent damage to the fistula.
- Patients should be advised to contact the hospital immediately if they notice bleeding, swelling or absence of bruit or thrill.
Cannulation of the arteriovenous fistula
In the first instance the fistula should be allowed 2–3 months to mature before cannulation is attempted, to allow healing of the anastomosis and some development of the vessels. After this time the fistula can be safely cannulated but the procedure should only be undertaken by experienced practitioners.
New fistulae may be prone to extravasation and clotting, which can be painful and distressing. Therefore, all attempts should be made to minimise trauma during the initial dialysis treatments when the fistula is still new and maturing.
If the patient is anxious, a local anaesthetic may be offered. Common forms of local anaesthetic include lignocaine or topical creams. Topical creams are a sensible choice; the injection of lignocaine can sting and defeat the object of pain-free cannulation. However, the topical cream needs to be applied at least 30 min before cannulation.
Prior to each cannulation a thorough physical examination of the fistula should be undertaken to check that there is no evidence of oedema, infection or bruising and that blood flow through the fistula is evident through the presence of the bruit and thrill.
The unit policy for strict asepsis and cleansing of the arm should be adhered to. Universal precautions should be employed, including wearing gloves, aprons and a face visor, as blood may splash into the face and eyes during cannulation. A tourniquet may be used prior to cannulation to help engorgement of the vessel but in well-established larger fistulae this is often unnecessary.
Cannulation protocols
Many units are now evaluating the use of planned protocols to insert fistula needles, with the aim of reducing complications and extending the longevity of the fistula. The effect of repeated puncture on fistulae can adversely affect elasticity of the surrounding tissue (Roy-Chaudhury et al. 2005). During each cannulation a small area of vessel tissue is displaced. When the needle is removed, this area is filled with a thrombus. Scar tissue is then formed, resulting in increased tissue and subsequent elongation of the vessel wall. Over time this results in a loss of elasticity of the vessel and dilation results in aneurysm formation and adjacent stenosis.
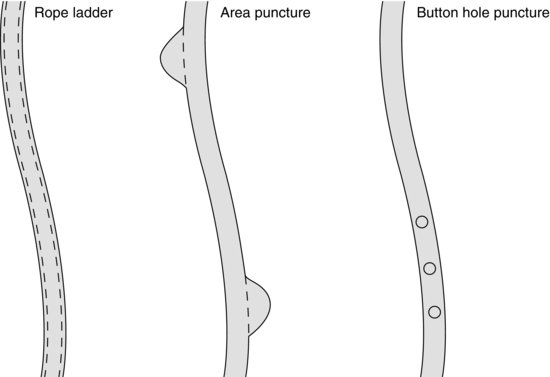
McCann et al. (2008) described three methods of cannulation and the effects of each (Figure 8.5).
- Rope ladder puncture: This describes the systematic use of the entire length of the vessel. Each needle is inserted at approximately 2 cm above the last site and back again, resulting in a uniform use of the vessel. This has demonstrated less aneurysm formation as the punctures per area are reduced (KDOQI 2006). However, care must be taken to ensure that an area puncture technique is not undertaken in the belief that the nurse is following the rope ladder technique.
- Area puncture: This describes the development and use of one or two areas of the fistula that are regularly used. This may result in increased aneurysm formation related to the number of repeated punctures over a small area causing increased tissue elongation and aneurysm formation. As a consequence of the increased risk of long term damage to the vascular system, area puncture is not advocated (Fluck and Kumwenda 2011).
- Button hole puncture: This describes the repeated puncture of exactly the same site at exactly the same angle into exactly the same hole each dialysis. Over time cylindrical scar tissue develops, guiding the needle into the right place. It is suggested that the button hole technique results in less aneurysm formation and has been shown to have high survival rates (Shearer et al. 2012). Button hole cannulation has also been associated with less pain associated with cannulation, and a reduction in haemostasis post dialysis (Sukthinthai et al. 2012).
There are no firm guidelines on whether the arterial needles should be placed antegrade (in direction of blood flow, away from the anastomosis) or retrograde (towards the hand). Brouwer (2005) and English (2005) advocate both positions. However Ozmen (2008) found no difference in the effectiveness of positioning on dialysis outcomes.
Cannulation skills can have an impact on the longevity of the vascular access. Poor cannulation techniques can lead to the increased use of central venous catheters or single needle dialysis (Van Loon 2009).
Whilst the adoption of protocols may be beneficial and ensure high standards of uniform practice, it is acknowledged that additional evaluation is necessary to provide evidence for the long-term effects of any of these protocols on the longevity of fistulae. The implementation of a vascular access monitoring programme can positively influence the longevity of vascular access (Van Loon 2007). Coupled with a cannulation training programme (Van Loon 2009) it can decrease access complications and increase staff competence and can be beneficial in prolonging the life of the vascular access. Both these initiatives are important in ensuring good standards of nephrology nursing practice.
Needle sets
The achievable blood flow for many patients is often underestimated. The careful selection of needles will ensure that optimum flows are obtained. Smaller needles (e.g. 16- and 17-gauge) produce high resistance as the blood pump is increased. This results in a ‘sucking’ effect on the needles and possible arterial alarms. Larger needles (14 g and 15 g) produce lower resistance and therefore avoid negative pressure as the blood pump speed is increased.
For patients with an AVF, needles should be inserted aseptically at 30–40° to the skin following appropriate skin preparation. Patients with an AVG should be cannulated at an angle of 45° to prevent damage to the material that the graft is made from.
The entire needle shaft should be inserted and the butterfly wing secured with tape. On insertion, a syringe should be attached to the end of the needle tubing and blood withdrawn. Any resistance to withdrawal may indicate that the needle position needs adjusting. Sometimes the needle hole is occluded against the side of the vessel wall. This can be corrected by placing a small piece of sterile gauze under the butterfly, this will lift the needle externally and lower the needle tip internally. If flow still cannot be obtained the presence of a clot may be suspected or there may be complete misplacement of the needle. In this event the needle should be removed. Arterial and venous needles need to be placed at least 5 cm apart to avoid recirculation.
If the needle punctures the wall of the vessel extravasation will occur, resulting in a painful visible swelling around the site. The needle should be removed and firm pressure applied for about 10 min before further insertion is attempted. On no account should the nurse push and pull the needle blindly, hoping that the needle will finally find the vessel. This will result in much pain and discomfort for the patient and will bruise and damage the surrounding tissue, which may in turn cause permanent damage to the fistula.
It is also important that nurses, no matter how experienced, recognise their limitations in relation to cannulation. If a cannulation attempt has failed after more than two or three attempts, assistance should be sought from a colleague, as the increased anxiety of the nurse and patient may negatively influence further attempts at successfully siting the needle.
Complications of arteriovenous fistulae
Defining a functioning vascular access can be problematic as there are no clearly accepted definitions. In the absence of a clear definition it could be argued that a functioning arteriovenous fistula (AVF) or arteriovenous graft (AVG) allows for repeated cannulation with two needles, allowing an adequate blood flow to deliver the prescribed dialysis dose.
Thrombosis
Thrombosis may occur in the immediate postoperative period or at a later date, sometimes following a hypotensive episode on dialysis (Chang et al. 2011), but is most commonly associated with stenosis of the AVF (KDOQI 2006). There are a number of different methods available for treating thrombosis, including surgical intervention, angioplasty and antithrombotics, or both. There are varying success rates. Surgical treatment (thrombectomy) may be indicated but salvage is often unsuccessful in the long term (Jain et al. 2008). However, if reported promptly, permanent damage may be avoided by the use of thrombolytic agents (KDOQI 2006).
Stenosis
Stenosis of the fistula can occur anywhere along its track, but is more common on the venous side of the fistula (Yevzlin, et al. 2009). However, irrespective of where it occurs there is an increased chance of the fistula failing. Stenosis at the anastamosis site will reduce blood flow into the fistula, increasing the risk of thrombosis.
A stenosis higher up above the needle site will increase pressure within the fistula, reduce blood flow out and increase the percentage of recirculation. All of which could adversely affect the adequacy of dialysis (KDOQI 2006).
The first choice of treatment for venous outflow stenosis is percutaneous angioplasty, but if the fistula is thrombosed then surgical intervention may be required (Fluck and Kumwenda 2011).
Aneurysm
Although unsightly, aneurysm formation in the AVF does not always adversely affect the function or blood flow through the fistula (Almehmi and Wang 2012). However, aneurysms are frequently associated with an increase in post dialysis haemostasis, pain and fistula loss (KDOQI 2006). Aneurysms can be caused by repeated area puncture (Figure 8.5). The skin eventually becomes much thinner as the aneurysms dilate (Almehmi and Wang 2012). Cannulation in the aneurysm should be avoided. Aneurysms are also associated with the development of stenosis of the vein above the needling site.
Steal syndrome
The creation of an AVF alters the normal blood flow through the lower part of the affected limb and hand. The creation of the AVF does not give rise to side-effects in the majority of patients, but approximately 5% can develop hand ischaemia in the affected hand (Scheltinga et al. 2009). The patient may complain of pain, oedema, coldness or ‘pins and needles’ as blood is ‘stolen’ from the hand as a result of the fistula (lower resistance in the arteriovenous anastomosis). Onset of steal syndrome can vary, with a rare acute onset within hours of surgery. Most patients develop symptoms over weeks, months, or even years. Surgical correction to restore blood supply to the hand is usually required, with subsequent loss to the fistula.
Infection
Infections of established arteriovenous fistulae are uncommon and are mainly localised to the immediate area, not progressing to bacteraemia. Treatment with antibiotic therapy is usually successful. Buttonhole cannulation has a higher infection rate then rope-ladder technique, therefore care must be taken to ensure that removal of the scab and skin disinfection is carried out carefully to reduce the risk (Birchenough 2010).
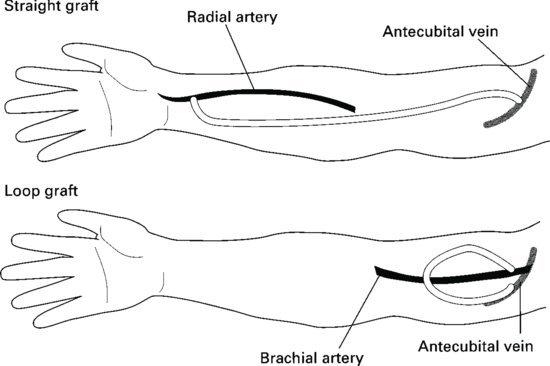
Arteriovenous grafts (Figure 8.6)
If the peripheral blood vessels are unsuitable for fistula formation the surgeon may decide to create a graft. Most grafts are created using synthetic materials such as polytetrafluoroethylene (PTFE). Grafts may be cannulated soon after insertion, preferably after 14 days.
The graft may be configured in a straight line or in a loop. Grafts are less compliant than fistulae, resulting in higher pressure through the vessels. Patients should be carefully taught how to care for the graft, especially with regards infection control.
The nurse should perform the same predialysis physical examination prior to cannulation. However, the angle of insertion of the needle is steeper than for an AVF and cannulation should be at an angle of 45°. Thorough skin preparation is vital as there is higher infection risk for grafts compared with fistulae. The arterial needle should be inserted at the arterial end of the graft at least 5 cm from the anastomosis site. The venous needle should be inserted in the venous end with the same considerations. For loop grafts it is important to identify the arterial and venous sides of the graft as incorrect needle placement will result in recirculation. Needles may be rotated through 90° following insertion (bevel of needle downwards), to reduce the risk of graft damage.
Monitoring access patency (surveillance)
Adequacy of dialysis is directly related to the total blood volume that is processed during the dialysis treatment. As such it is important that the access used should be capable of delivering the optimum blood flow. However, stenosis occurring in the anastamosis area will reduce the volume of blood passing through the fistula; while a stenosis above the needling site will likewise decrease blood flow and increase the chance of recirculation. Regular monitoring of the blood flow will provide valuable information and trends on blood flow through the fistula and the percentage of recirculation.
There are a number of methods that can be used to monitor the effectiveness of the vascular access. These can range from low technology, simple observation and assessment of the access each dialysis session through to high technology solutions that measure blood flow via ultrasonic methods.
Physical examination of the vascular access at each dialysis session is a simple and cost effective method of identifying changes over time. The ‘rule of six’ is a simple method and is summarised in Box 8.1. There are three components to assessment of the vascular access: inspection (look), palpation (feel) and ausculation (listen). The AVF or AVG should not have any indications of infection or inflammation. On palpation the fistula will have a soft continuous thrill along it length and should be easily compressible (Vachharajani 2012). On auscultation the AVF should have a low-pitched continuous bruit.
- A minimum of 6 mm in diameter with discernible margins when a tourniquet is in place
- Less than 6 mm deep below the surface.
- Have a blood flow greater than 600 ml/minute.
- Have at least 6 cm of straight segment for cannulation.
As well as a physical examination, monitoring changes in venous and arterial pressure during dialysis over time can also provide meaningful information on the state of the vascular access. Increasing venous pressure over a period of weeks or months may indicate a stenosis, especially if associated with prolonged bleeding times post dialysis. A reduction in achievable blood flow over a period of time could be an indication that the blood flow through the vascular access is falling. An unexplained drop in the patient’s Kt/V can also provide clues to the condition of the vascular access, and may indicate an increase in recirculation.
Surveillance of the blood flow through the fistula is another method of assessing the health of vascular access. Blood flow through the fistula or graft of less than 600 ml/min is an indicator of access failure (KDOQI 2006; Fluck and Kumwenda 2011). There are a number of methods of indirectly measuring the blood flow, however, there is no single technique that has been shown to be superior (Fluck and Kumwenda 2011).
Vascular access standard
The Renal Association (Fluck and Kumwenda 2011) has recommended that 65% of all patients starting HD for the first time should commence with an AV fistula and 85% of all patients on maintenance haemodialysis should have a functioning AV fistula. If this is to be achieved then there will be increasing pressure on nurses to care for AVF proactively and to ensure that only well-trained competent nurses carry out cannulation. Detailed ways in which the multiprofessional team can work together to achieve this aim are beyond the scope of this book, but McCann et al. (2008) provide a good review of how to prolong vascular access patency.
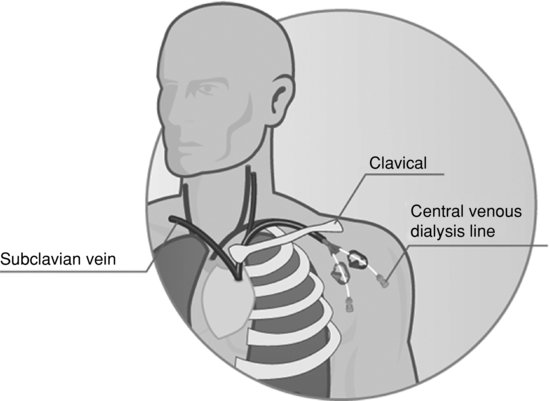
Percutaneous access
Percutaneous access is the term used to describe the insertion of a cannula or catheter into a major vein, often termed a central venous catheter (CVC) (Figure 8.7). Catheters may be inserted as a temporary measure, as in acute kidney injury, or for temporary use whilst a fistula matures. Potential sites include the subclavian, femoral and internal jugular veins. The use of the subclavian vein is not recommended in patients with end-stage kidney disease as this may adversely affect the success of the creation of an AVF due to central venous stenosis. Femoral catheters should only be used in those who are immobile and should be changed every 1–3 days. General knowledge and understanding of central venous catheters (CVCs) as a method of haemodialysis vascular access can be gained by reading McCann et al. (2010)
More commonly, percutaneous catheters are being inserted as permanent access for patients whose fistulae have either failed or whose vessels are inadequate to attempt AVF creation in the first place. The permanent catheters are cuffed and inserted through the creation of a subcutaneous tunnel, as this ensures optimal placement of the catheter and helps reduce the rate of infection.
Catheters are invariably double-lumen, but single-lumen catheters are occasionally used. Catheters may be inserted under general or local anaesthetic and nursing care pre- and postoperatively will be the same as for any surgical procedure. After insertion it is essential that the correct placement of the catheter is checked by X-ray prior to dialysis as insertion complications may include pneumothorax and puncture of the adjacent vessels.
The nurse’s responsibility includes the maintenance of catheter patency, patient education, prevention of infection and early intervention when infection occurs. Nurses caring for percutaneous access must demonstrate meticulous care, as defined by a strict unit policy. This policy should include the need for strict asepsis during any catheter intervention (Vanholder et al. 2010). The exit site should be examined before each dialysis and observed for signs of infection such as soreness, redness or the presence of exudate. Exit sites should be covered with a dressing that will maintain an optimum environment conducive to healing (not too wet and not too dry) and one that will repel Staphylococcus aureus.
The Renal Association has recommended an audit benchmark of an annual Staphylococcus aureus bacteraemia rate of less than 2.5 episodes per 100 patients on HD and less than 1.0 episode per 100 patients for meticillin resistant Staphylococcus aureus (MRSA) over 2 years (Fluck and Kumwenda 2011).
Chlorhexidine aqueous solution or povidone-iodine can be used to clean the exit site and then a nonocclusive dressing is applied (Pratt et al. 2007). It is important to note that manufacturers’ guidelines on use of a cleansing agent should be strictly followed, as chlorhexidine alcohol solutions can degrade the materials of which the central line are made. The catheter hubs and portals are common sites of infection and should be cleaned prior to connection and disconnection from dialysis (Pratt et al. 2007).
Maintaining patency of the central venous catheter
To maintain patency between dialysis treatments it is common to instil a bolus of heparin (usually 5000 iu/ml) equal to the volume of each catheter lumen. It is important that the exact volume of each respective lumen is ascertained to prevent giving a systemic dose of heparin to the patient. However, other locks such as citrate or a mixture of heparin and gentamycin have been found to be equally effective (Betjes and Van Agteren 2004; McIntyre et al. 2004). The use of an antimicrobial lock has the advantage of reducing catheter related infections (Vanholder et al. 2010), but can be more expensive to use than heparin. This modest increase in price though can be a cost saving when taking into account treatment costs for blood stream infections.
There has been an increase in the use of trisodium citrate as a catheter lock in recent years, and is this is now considered a safe alternative to heparin, especially as the price differentiation between the two has decreased. The use of trisodium citrate has been shown to have improved outcomes with regards catheter exchange, tissue plasminogen activator (tPA) (thrombolytic) use and access-related hospitalisations compared with heparin locking (Lok et al. 2007). Two recent meta-analyses have shown that the use of trisodium citrate has a lower incidence of catheter related blood stream infections when compared with heparin as a lock (Labriola et al. 2008; Yahav et al. 2008).
Before the next dialysis treatment, the anticoagulant lock must be removed by aspirating the catheter with a syringe and then flushing with 0.9% normal saline before connecting the dialysis lines. Nevertheless, clotting is a common complication, either preventing the use of the catheter, or reducing flows rates adversely, affecting adequacy of the dialysis. Several attempts may be needed to aspirate a clot in the catheter if resistance is felt. It is vital that any clot is removed and no attempt is made to flush a catheter that cannot be aspirated from either lumen. In permanent catheters, the administration of urokinase should dissolve the clot if all other methods have failed, and should be left for at least 30 min before or between dialysis sessions.
In dual-lumen catheters it is important to use the arterial and venous lumens appropriately. Occasionally, flow from the arterial lumen is partially occluded owing to poor positioning of the arterial holes against the side of the vessel wall. The lines may have to be reversed to achieve an acceptable blood flow, however it must be borne in mind that reversal of the lines will result in increased recirculation (McCann et al. 2010).
Haemodialysis Equipment
The dialyser
The dialyser is the functional unit of the extracorporeal circuit just as the nephron is the functional unit of the kidney, and some patients and nurses refer to the dialyser as the ‘kidney’.
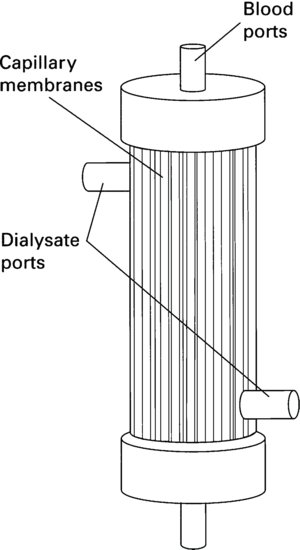
Manufacturers have made significant advances in the development of membranes that provide highly efficient clearance of waste products and which are biocompatible for the patient. There are two types of dialyser design: the hollow fibre (Figure 8.8) and the parallel plate. However, the vast majority of dialysers produced and used today are hollow fibre dialysers.
The hollow fibre dialyser is made up of thousands of hollow fibres or capillaries about the thickness of a human hair. The fibres are secured at each end of the cylindrically shaped dialyser in a polyurethane potting compound. Blood passes through the centre of each fibre like a straw, whilst the dialysate passes on the outside of the fibres in the opposite direction.
The plate dialyser consists of sheets of membranes arranged in layers. Blood passes through the space between one set of layers whilst the dialysate passes through the adjacent layers in the opposite direction. Plates have more compliance (stretch) than hollow fibre dialysers and therefore have higher priming volumes.
The dialyser membrane
The choice of membrane type is becoming increasingly important as part of the patient’s individual dialysis prescription. In addition to selecting the membrane which provides the desired clearance and fluid removal, the nurse should also consider the issue of biocompatibility related to the patient’s needs.
A large and increasing variety of membrane types are available, but these fall broadly into three categories:
- Cellulose membrane (e.g. Cuprophan): low flux
- Modified cellulose membranes (e.g. cellulose acetate, cellulosynthetic such as Hemophan, cellulose triacetate): mid or high flux
- Synthetic membranes (e.g. polysulfone, polyacrilynitrile (PAN), polymethylmetacrylate (PMMA), polycarbonate (Gambrane) and polyamide): mid- or high flux
The efficiency with which a membrane clears water and solutes is described as its flux properties. Thin membranes with large pores are very permeable to water and large molecules and are called high-flux membranes as they have the ability to clear solutes with a molecular weight of up to 30 kDa. Low-flux membranes are less permeable to water and solutes but will provide adequate clearance of solutes up to a molecular weight 10 kDa. The membrane’s permeability to water is described as its ultrafiltration coefficient (KUf), and is measured in ml/h/mmHg (the number of millilitres per hour of fluid that is removed for every one unit of pressure across the membrane). The efficiency of solute clearance is measured by the mass transfer urea coefficient or KoA, indicating the effectiveness of the membrane for allowing solute to pass across its surface.
High-flux membranes have a high KUf (>10) and moderate-water-permeability membranes have a KUf of between 5 and 10 ml/h/mmHg. Details of the properties of each dialyser can be found on the dialyser specification sheet which is provided with each box of dialysers. The use of high-flux membranes during haemodialysis and haemodialfiltration is now the membrane of choice (Tattersall et al. 2010). The routine use of high-flux membranes has been found to have a positive effect on the survival of patients on haemodialysis, especially at risk patients with low serum albumin (Locatelli et al. 2009b).
Solute removal by modified cellulose and synthetic membranes is similar, although β2-microglobulin clearance is greater with synthetic membranes, and this may translate into fewer amyloid dialysis deposits (Schwalbe et al. 1997).
Biocompatibility
It is ironic that the very process of haemodialysis, whilst providing an effective treatment for established renal failure, can result in significant side effects due to the interaction of the blood with the various components that make up dialysis equipment. The process of dialysis requires the repeated exposure of the patient’s blood to foreign substances including the dialyser membrane, blood lines, dialysate, chemicals, drugs and water. Biocompatibility can be defined as the use of a material that elicits the least amount of inflammatory response during dialysis.
At its simplest, the exposure of blood to the artificial surfaces of the dialysis circuit results in coagulation within the dialyser and dialysis line. Although all membranes elicit an increased incidence of thrombogenesis and fibrinolysis, cellulosic membranes create a more marked increase than synthetic membranes (De Sanctis 1996). However, dialysis may also initiate subtle but long-term effects on immunological responses that include activation of the complement system and release of cytokines involved in the inflammatory process and anaphylactic reactions.
Recent moves to ensure that the products used during dialysis (the dialyser and dialysis lines) are sterilised through gamma irradiation has reduced one serious issue of bio-incompatibility, namely the incidence of ‘first use syndrome’ associated with sterilisation using ethylene oxide. Although rare, patients can experience a severe reaction to a dialyser membrane (Coentrao et al. 2010), therefore when exploring unexplained incidents on dialysis the possibility of a membrane reaction should be considered.
Dialysis-related amyloidosis leading to conditions such as carpal-tunnel syndrome is associated with elevated β-2 microglobulin (β-2M) levels. This was initially thought to be related to exposure of cellulose membranes, however subsequent studies have found no significant evidence that bioincompatible membranes have a marked increase on the production of β-2M. Interestingly, biocompatible high-flux dialyser membranes have been shown to increase the clearance of β-2M, slowing the development of amyloidosis (Traut et al. 2005). More focus is now on the use of high-flux dialysis and haemodiafiltration as the treatment of choice for renal replacement therapy.
Other more subtle effects of biocompatibility should not be ignored. It has also been suggested that bioincompatible membranes adversely affect the nutritional parameters of patients in comparison to those dialysed with biocompatible membranes.
Poor water quality can also induce inflammatory reactions. Bacterial and endotoxin contamination resulting from biofilm development have been implicated in increased morbidity of haemodialysis patients (Hoenich and Levin 2003; Lonnemann 2004). Standards aim to define safe levels of chemical and bacteria contaminants, outlining regular monitoring to identify potential quality problems (Mactier et al. 2011) although there are calls for international harmonisation (Nystrand 2009).
The importance of water quality cannot be emphasised enough in units using high-flux or haemodialfiltration. The larger pores in the dialysis membrane increase the risk of exposure to bacteria or endotoxin. Therefore the risk of backfiltration during dialysis always exists, and poor water quality will adversely affect the patient.
Water treatment
A patient dialysing 4 h, three times a week is exposed during treatment to approximately 6240 L of water a year. Because of this level of exposure, the water used during dialysis must be well controlled and regularly monitored for impurities and contamination. Raw water coming into the dialysis unit from the mains supply contains many contaminants that pose a potential risk to the patient. The clinical effects on patients caused by various contaminants can be seen in Table 8.1.
Table 8.1 Clinical effects of water contaminants.
| Symptom | Related contaminants |
| Anaemia | Aluminium, chloramine, nitrate, lead, copper, zinc |
| Bone disease | Aluminium, fluoride, |
| Hypertension | Calcium, magnesium, sodium |
| Hypotension | Bacteria, endotoxin, nitrate |
| Acidosis | Low pH, sulphate |
| Muscle weakness | Calcium, magnesium |
| Nausea/vomiting | Bacteria, endotoxin, chloramine, low pH, nitrate, sulphate, calcium, magnesium, copper, zinc |
| Neurological disturbance | Aluminium, lead, calcium, magnesium |
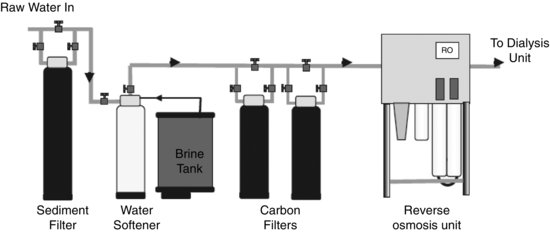
In order to avoid these symptoms, raw water is treated to reduce the concentration of contaminants to safe levels. The standards controlling contaminant concentration of processed water used are defined by the Association for the Advancement of Medical Instrumentation (2004) and ISO (2011). A simplified schematic drawing of a typical water-treatment plant can be seen in Figure 8.9.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree