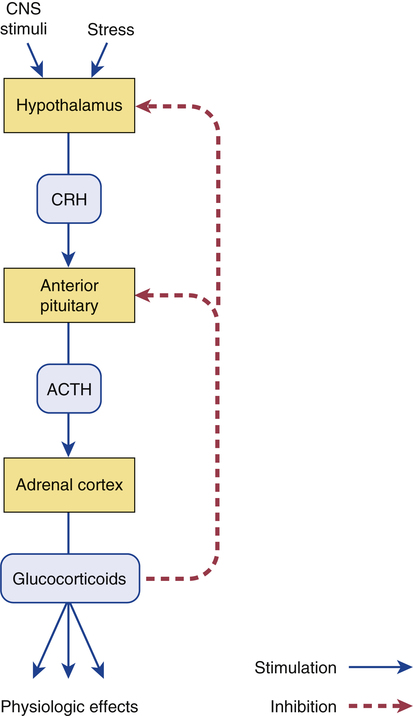
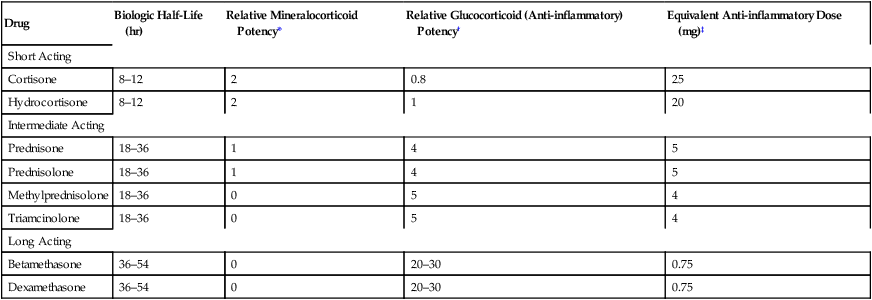
CHAPTER 72 As implied by the chapter title, glucocorticoids have both endocrine and nonendocrine applications. In low (physiologic) doses, glucocorticoids are used to treat adrenocortical insufficiency. In high (pharmacologic) doses, glucocorticoids are used to treat inflammatory disorders (eg, asthma, rheumatoid arthritis) and certain cancers and to suppress immune responses in organ transplant recipients. The endocrine applications of the glucocorticoids are discussed in Chapter 60. Nonendocrine uses are discussed here. Physiologic responses can be elicited with low doses of glucocorticoids. At higher doses, these effects are simply more intense. When glucocorticoids are used to treat nonendocrine disorders, physiologic responses occur as side effects. Physiologic effects are discussed at length in Chapter 60, and hence discussion here is brief. To varying degrees, individual glucocorticoids can exert actions like those of aldosterone, the major mineralocorticoid released by the adrenals. Accordingly, glucocorticoids can act on the kidney to promote retention of sodium and water while increasing urinary excretion of potassium. The net result is hypernatremia, hypokalemia, and edema. Fortunately, most of the glucocorticoids employed as drugs have very low mineralocorticoid activity (Table 72–1). TABLE 72–1 Systemic Glucocorticoids: Half-Lives, Relative Potencies, and Equivalent Doses *Relative mineralocorticoid activity (sodium and water retention; potassium depletion): 0 = very low, 1 = moderate, 2 = high. †Glucocorticoid potency values are relative to the potency of hydrocortisone. ‡Approximate oral or intravenous dose needed to produce equivalent anti-inflammatory effects. Synthesis and release of glucocorticoids are regulated by a negative feedback loop. The principal components of the loop are the hypothalamus, anterior pituitary, and adrenal cortex (Fig. 72–1). The loop is turned on when stress or some other stimulus from the central nervous system acts on the hypothalamus to cause release of corticotropin-releasing hormone (CRH). CRH then stimulates the pituitary to release adrenocorticotropic hormone (ACTH), which in turn acts on the adrenal cortex to promote synthesis and release of cortisol (the principal endogenous glucocorticoid). Cortisol has two basic effects: first, it stimulates physiologic responses; second, it acts on the hypothalamus and pituitary to suppress further release of CRH and ACTH. By inhibiting release of CRH and ACTH, cortisol suppresses its own production. As a result, this negative feedback loop keeps glucocorticoid levels within an appropriate range. When glucocorticoids are administered chronically in large doses, the feedback loop remains continuously suppressed. As discussed later, persistent suppression can be dangerous. It is important to appreciate that the mechanisms by which glucocorticoids suppress inflammation are more diverse than the mechanisms by which nonsteroidal anti-inflammatory drugs (NSAIDs) act. As discussed in Chapter 71, NSAIDs suppress inflammation primarily by inhibiting prostaglandin production. The glucocorticoids share this mechanism and act in other ways too. Because they act by multiple mechanisms, glucocorticoids have much greater anti-inflammatory effects than do NSAIDs. Glucocorticoids are used to treat endocrine disorders and nonendocrine disorders. Endocrine disorders (eg, Addison’s disease) can be managed with low-dose therapy and are considered in Chapter 60. Nonendocrine applications, which require much higher doses, are discussed here. Because prolonged, high-dose therapy can produce serious adverse effects, the potential benefits of treatment must be weighed carefully against the very real risks. Glucocorticoids can control symptoms of allergic reactions. Responsive conditions include allergic rhinitis (see Chapter 77), bee stings, and drug-induced allergies. Because glucocorticoid responses are delayed, these drugs have little value as acute therapy for severe allergic reactions (eg, anaphylaxis). For life-threatening allergic reactions, epinephrine is the treatment of choice. Glucocorticoids are the most effective antiasthma agents available. For treatment of asthma, they may be administered orally or by inhalation. Adverse effects are minimal with inhaled glucocorticoids. In contrast, oral therapy can cause serious toxicity, and hence should be reserved for patients who have failed to respond to safer treatments (eg, inhaled glucocorticoids, inhaled beta2-adrenergic agonists, inhaled cromolyn sodium). The use of glucocorticoids in asthma is discussed at length in Chapter 76. Glucocorticoids are beneficial in a wide variety of skin diseases, including pemphigus, psoriasis, mycosis fungoides, seborrheic dermatitis, contact dermatitis, and exfoliative dermatitis. For mild disease, topical administration is usually adequate. For severe disorders, systemic therapy may be needed. It should be noted that topical glucocorticoids can be absorbed in amounts sufficient to produce systemic toxicity. Topical therapy is discussed further in Chapter 105. Glucocorticoids are used in conjunction with other anticancer agents to treat acute lymphocytic leukemia, Hodgkin’s disease, and non-Hodgkin’s lymphomas. Benefits derive from the direct toxicity of glucocorticoids to malignant lymphocytes. Treatment kills lymphoid cells and causes regression of lymphatic tissue. The use of glucocorticoids to treat cancers is discussed further in Chapter 103. Glucocorticoids, together with other immunosuppressant agents, are used to prevent rejection of organ transplants. Glucocorticoids are initiated at the time of surgery and continued indefinitely. The use of glucocorticoids for immunosuppression is discussed further in Chapter 69. Preterm infants are at high risk of respiratory distress syndrome. Why? Because their adrenals cannot produce the glucocorticoids needed for lung maturation. When preterm delivery is imminent, injecting the mother with glucocorticoids (usually dexamethasone or betamethasone) reduces the risk of neonatal respiratory distress. Steroids may also reduce the incidence of intraventricular hemorrhage and necrotizing enterocolitis (inflammation of the small intestine and colon). Antenatal use of glucocorticoids is discussed further in Chapter 107. Drugs can help reduce bone loss. All patients should receive calcium and vitamin D supplements. Sodium restriction combined with a thiazide diuretic can enhance intestinal absorption of calcium and can decrease urinary excretion of calcium. There is solid evidence that a bisphosphonate can prevent glucocorticoid-induced bone loss. Dosing may be done orally (eg, risedronate [Actonel], 5 mg/day) or by IV infusion (eg, zoledronate [Reclast], 5 mg once a year). How do bisphosphonates help? They inhibit bone resorption by osteoclasts. Calcitonin [Miacalcin, others], which also inhibits osteoclasts, is another option. For patients with significant bone loss, teriparatide [Forteo] may be preferred. Why? Because, unlike bisphosphonates and calcitonin, which only prevent bone resorption, teriparatide actively promotes new bone formation. In postmenopausal women, estrogen therapy is an effective way to reduce bone loss. However, as discussed in Chapter 61, the risks of estrogen therapy generally outweigh the benefits. The roles of calcium, vitamin D, bisphosphonates, calcitonin, teriparatide, and estrogen in the prophylaxis and treatment of osteoporosis are discussed fully in Chapter 75.
Glucocorticoids in nonendocrine disorders
Review of glucocorticoid physiology
Physiologic effects
Effects on water and electrolytes.

Drug
Biologic Half-Life (hr)
Relative Mineralocorticoid Potency*
Relative Glucocorticoid (Anti-inflammatory) Potency†
Equivalent Anti-inflammatory Dose (mg)‡
Short Acting
Cortisone
8–12
2
0.8
25
Hydrocortisone
8–12
2
1
20
Intermediate Acting
Prednisone
18–36
1
4
5
Prednisolone
18–36
1
4
5
Methylprednisolone
18–36
0
5
4
Triamcinolone
18–36
0
5
4
Long Acting
Betamethasone
36–54
0
20–30
0.75
Dexamethasone
36–54
0
20–30
0.75
Control of synthesis and secretion
Pharmacology of the glucocorticoids
Molecular mechanism of action
Pharmacologic effects
Anti-inflammatory and immunosuppressant effects
Therapeutic uses in nonendocrine disorders
Allergic conditions.
Asthma.
Dermatologic disorders.
Neoplasms.
Suppression of allograft rejection.
Prevention of respiratory distress syndrome in preterm infants.
Adverse effects
Osteoporosis.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree