Introduction
 Clinical Presentation and Classification
Clinical Presentation and Classification
Maintenance of Fluid and Electrolyte Balance
Measures to Minimize Fistula Output
Definition of the Fistula Tract
Skin Protection and Containment
 Fistula Management for the WOC Nurse
Fistula Management for the WOC Nurse
Progress toward/Impediments to Spontaneous Closure
Abdominal Contours and Fistula Opening
Interventions and Containment Strategies
Absorptive Dressings and Moisture Barriers
Negative Pressure Wound Therapy
Education and Emotional Support
 Vesicovaginal, Rectovaginal, or Enterovaginal Fistulas
Vesicovaginal, Rectovaginal, or Enterovaginal Fistulas
Introduction
A fistula (plural fistulas or fistulae) is an abnormal passage between two or more epithelialized surfaces that results in communication between one body cavity or hollow organ and another hollow organ or the skin (Bryant & Best, 2015). An enterocutaneous fistula (ECF) refers to an opening from the intestine to the skin. Although ECFs can be located within a wound, they should not be confused with a draining wound, surgically placed drain site, or wound dehiscence.
The mortality rates for patients with ECFs range from as low as 5.5% to as high as 30%; death is most often due to sepsis, malnutrition, or fluid and electrolyte imbalance (Kaur & Minocha, 2000; Li et al., 2003; McNaughton et al., 2010). Although the true incidence of ECF development is unknown, Teixeira et al. (2009) reported an incidence of 1.5% in a large study involving 2,373 trauma patients who required laparotomy. They also found that patients with ECFs required significant hospital resources with a statistically significant increase in intensive care unit length of stay (28.5 ± 30.5 vs. 7.6 ± 9.3 days, p = 0.004), hospital length of stay (82.1 ± 100.8 vs. 16.2 ± 17.3 days, p < 0.001), and mean hospital charges ($539,309 vs. $126,996, p < 0.001).
An interdisciplinary team is needed to meet the needs of the patient with a fistula (Canadian Association for Enterostomal Therapy [CAET], 2009). Most authors suggest that essential team members include wound/ostomy nurse, dietitian, pharmacist, nurse, social worker, surgeon, and physician (Haffejee, 2004; Hollington et al., 2004; Lal et al., 2006). Other team members include pain specialists, radiologists, physiotherapists, and occupational therapists (Lal et al., 2006; Oneschuk & Bruera, 1997). ECF management is one of the most difficult clinical challenges for the wound/ostomy nurse. It can also be the most rewarding experience once the patient’s quality of life is restored through individualized, unique, and best practice interventions.
Clinical Presentation and Classification
Fever and abdominal pain are the initial indicators of a possible fistula (Nussbaum & Fischer, 2006); however, these are nonspecific indicators. The definitive indicator of a cutaneous fistula is the passage of gastrointestinal (GI) secretions or urine into an open wound bed or through an unintentional opening onto the skin. Manifestations of a fistula tract terminating in the vagina include passage of urine (vesicovaginal fistula) or passage of gas, feces, and/or purulent and extremely malodorous drainage (rectovaginal or enterovaginal fistula). Irradiation-induced rectovaginal fistulas often are preceded by diarrhea, passage of mucus and blood rectally, a sensation of rectal pressure, and a constant urge to defecate (Saclarides, 2002). Fistulas between the intestinal tract and the urinary bladder (e.g., colovesical fistula) present with passage of gas or stool-stained urine through the urethra.
CLINICAL PEARL
The definitive indicator of a cutaneous fistula is the passage of GI secretions or urine into an open wound bed or through an unintentional opening onto the skin.
The pH of the effluent may suggest the origin of the fistula tract. For example, extremely acidic fluid (pH 1.0 to 3.0) suggests a gastric fistula, whereas highly alkaline output (7.8 to 8) is consistent with a pancreatic fistula (Huether, 2002).
Most clinicians describe and classify fistulas according to location, involved structures, and volume of effluent. Although less frequently used, fistulas may also be classified by complexity (see Table 17-1). Mucous fistulas are surgically created openings into the defunctionalized section of bowel; they secrete mucus only, which is relatively easy to contain with dressings or pouches, and they do not increase morbidity or mortality. They are therefore not further discussed in this chapter.
TABLE 17-1 Fistula Classification
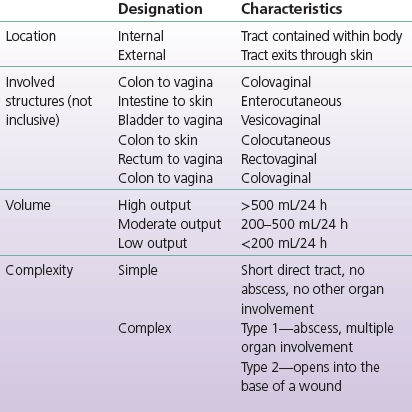
Modified from Bryant, R., & Best, M. (2015). Management of draining wounds and fistulas. In R. Bryant & D. Nix (Eds.), Acute and chronic wounds: Current management concepts (5th ed.). St. Louis, MO: Mosby. (In Print.)
CLINICAL PEARL
Fistulas are typically “named” for the organ of origin and the organ of termination; for example, an ECF is one from the bowel to the skin, and a colovesical fistula is one from the colon to the vagina.
Etiologic Factors
ECFs commonly develop postoperatively, due to anastomotic breakdown, but can also occur spontaneously, as a result of inflammatory bowel disease, cancer, or diverticulitis. The risk of fistula formation is further increased when one of these conditions is complicated by malnutrition, sepsis, hypotension, vasopressors, or corticosteroids (Nussbaum & Fischer, 2006).
Approximately 25% of fistulas develop spontaneously and are associated with an intrinsic intestinal disease (cancer, radiation, diverticulitis, inflammatory bowel disease, appendicitis) or external trauma. Spontaneous fistulas are generally resistant to spontaneous closure. Patients treated for a pelvic cancer are particularly vulnerable to ECFs due to radiation damage; the fistula may develop immediately following radiation or years later (Tran & Thorson, 2008). An analysis of 41 publications reported that 17% of patients receiving pelvic radiation developed ECFs, and the average time frame for fistula development was 3.4 years following completion of radiation therapy (Meissner, 1999). Irradiation-induced ECFs are more likely to occur in patients who receive higher radiation doses (>5,000 cGy), smoke cigarettes, or have atherosclerosis, hypertension, diabetes mellitus, advanced age, pelvic inflammatory disease, or previous pelvic surgery (Hollington et al., 2004; Saclarides, 2002; Tran & Thorson, 2008).
CLINICAL PEARL
Most ECFs occur postoperatively, as a result of anastomotic breakdown; however, fistulas may also develop spontaneously as a result of inflammatory bowel conditions (e.g., diverticulitis, Crohn’s disease, radiation enteritis) or trauma.
The majority of ECFs (75% to 85%) are iatrogenic (inadvertently induced from a medical procedure); these fistulas develop postoperatively due to anastomotic breakdown (Nussbaum & Fischer, 2006). A key risk factor for anastomotic breakdown is malnutrition (Mäkelä et al., 2003; Telem et al., 2010). Additional risk factors for postoperative ECF development include existing conditions such as inflammatory bowel disease, cancer, or previous radiation therapy. Surgery-related risk factors include inadequate blood supply, poor suture technique, inadequate bowel prep (e.g., emergency surgery), extensive lysis of adhesions, and trauma surgery (Kassis & Makary, 2008; Nussbaum & Fischer, 2006; Wong et al., 2004). The method of anastomosis (stapled or hand-sewn) has not proven to be a predictor of ECF after surgery for trauma (Demetriades et al., 2002; Kirkpatrick et al., 2003). Patients scheduled for elective surgical procedures should receive adequate nutrition preoperatively in order to minimize the risk of anastomotic breakdown. When emergency surgery is necessary, prevention strategies include adequate intravenous fluids, circulatory support, keeping the patient warm, and broad-spectrum antibiotics (Kassis & Makary, 2008; Maykel & Fischer, 2003). If there are concerns regarding delayed healing of the intestinal anastomosis due to poorly controlled morbidities and extensive intra-abdominal infection, a temporary stoma may be created proximal to the anastomosis to protect the anastomosis during healing; the stoma is closed once healing is complete. In the past, temporary stomas were commonly performed following bowel resection and anastomosis due to traumatic injury; this is no longer standard, as most of these anastomoses have been shown to heal in a timely manner. Interestingly, recent studies indicate that diversion following colonic anastomosis for penetrating colonic injury did not reduce the incidence of septic complications, including abscess and fistula (Demetriades et al., 2001).
Medical Management
Management for this patient population requires a clear understanding of the underlying pathophysiology, astute assessment skills, knowledge about management alternatives and options, competent technical skills, diligent follow-up, and persistence. A comprehensive and effective interdisciplinary approach is required to reduce complications and achieve closure (Bryant & Best, 2015). Spontaneous closure of an ECF is defined as closing with medical management within 6 to 8 weeks (Teixeira et al., 2009). Wong et al. (2004) report 90% of simple type 1 fistulas close spontaneously, whereas <10% of complex type 2 fistulas close spontaneously. Additional factors that correlate with spontaneous closure include postoperative occurrence, low output, absence of sepsis, and adequate nutrition (Campos et al., 1999; Nussbaum & Fischer, 2006). When sepsis is controlled and appropriate nutritional support is provided, approximately 19% to 40% of all fistulas close spontaneously with medical management. The majority of ECFs that close spontaneously do so within 5 weeks (Kassis & Makary, 2008).
CLINICAL PEARL
Only a limited percentage (19% to 40%) of fistulas close spontaneously, even with optimal management; those that do close spontaneously usually do so within 5 to 6 weeks.
Objectives of ECF management are described below and include: (1) maintenance of fluid and electrolyte balance; (2) measures to minimize fistula output; (3) control of infection; (4) nutritional support; (5) definition of the fistula tract; and (6) skin protection and containment of effluent.
Maintenance of Fluid and Electrolyte Balance
Each day 8 to 10 L of fluid flows through the jejunum, depending on oral intake. In the intact functioning intestine, 98% of this fluid is (re)absorbed, leaving only 100 to 200 mL of fluid to be excreted in the stool. Development of a fistula permits abnormal fluid losses, with volume of loss determined in part by size of the fistulous opening and in part by anatomic location within the bowel. For example, fistulas located in the proximal small bowel are generally high output, while fistulas occurring in the colon are typically low output. When providing fluid replacement, the prescribing provider must consider both the volume and the composition of the fistulous drainage, both of which are impacted by fistula location within the GI tract. Severe metabolic disturbances have been noted with ECF output >200 mL/day due to loss of hydrogen, chloride, sodium, and potassium ions (Arebi & Forbes, 2004; Makhdoom et al., 2000). Careful monitoring of tissue perfusion, weight, urine, and fistula output is necessary to evaluate fluid balance. Adequate fluid and electrolyte replacement is critical to prevent hypovolemia and circulatory failure in the patient with a high-output ECF (Makhdoom et al., 2000).
Measures to Minimize Fistula Output
A key intervention for promotion of spontaneous closure is to minimize the amount of fluid flowing through the fistula tract, that is, to reduce oral and enteral intake. This may be done by making the patient NPO (nothing by mouth), or by limiting oral and/or enteral intake to the amount needed to keep the intestinal mucosa healthy. Significantly reduced oral/enteral intake minimizes fistula output by decreasing luminal contents, GI stimulation, and pancreaticobiliary secretion. Administration of H2 antagonists (e.g., cimetidine), frequently used to prevent stress ulcerations, also decreases gastric, biliary, and pancreatic secretions. Despite this reduction in secretions, H2 receptors have not been shown to affect either the number of ECFs that close spontaneously or the time to ECF closure (Arebi & Forbes, 2004; Evenson & Fischer, 2006).
Somatostatin and its analog, octreotide, are known to decrease intestinal output in some situations, and have been used as adjunctive therapy in the treatment of ECF. Somatostatin is administered through continuous intravenous infusion due to its short half-life of 1 to 2 minutes. Octreotide’s half-life is almost 2 hours, and it is administered three times daily subcutaneously (Makhdoom et al., 2000). In the past, there was lack of consensus as to whether these medications increased closure rates or decreased time to closure (Arebi & Forbes, 2004; Fagniez & Yahchouchy, 1999; Hesse et al., 2001; Sancho et al., 1995; Torres et al., 1992). More recently, a systematic review and meta-analysis conducted by Coughlin et al. (2012) concluded that somatostatin analogs appear to decrease the duration of ECFs (time to closure) and hospital stays, though there was no reduction in number of fistulas that closed and no reduction in fistula-related mortality. Octreotide is not recommended for routine use due to reports of precipitated villous atrophy, interruption of intestinal adaptation, and acute cholecystitis. Some authors recommend a 5- to 8-day trial, with discontinuation of the octreotide if there is no significant reduction in fistula output within that time frame (Draus et al., 2006).
CLINICAL PEARL
Measures to promote spontaneous closure of ECFs include nutritional support, limited oral/enteral intake to reduce the volume of fistula output, and possibly negative pressure wound therapy (NPWT) (to promote wound healing).
Control of Infection
Uncontrolled sepsis and sepsis-associated malnutrition have been shown to be important determinants of mortality in the patient with an ECF (Dubose & Lundy, 2010; Lynch et al., 2004). Symptoms may include localized and then diffuse abdominal pain, ileus, and fever. Presence of abscess can be detected with computed tomographic scanning or ultrasound. CT-guided drainage is the initial management of choice in patients presenting with spontaneous or postoperative intra-abdominal abscess. This can obviate the need for early operative intervention. As seen in Figure 17-1, if a fistula develops, a definitive procedure can be deferred with the drain left in place to control further abscess formation (Davis et al., 2000; Lynch et al., 2004). Abscess contents should be cultured after percutaneous or surgical drainage, to assure appropriate antibiotic therapy (Wong et al., 2004).
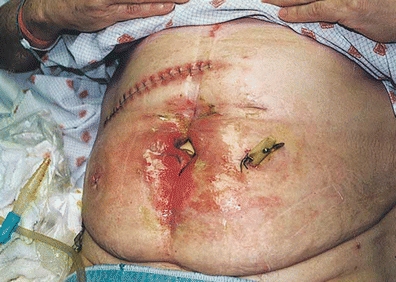
FIGURE 17-1. ECF (small bowel to skin); extensive skin damage due to enzymatic drainage; drain in place for abscess management.
CLINICAL PEARL
Somatostatin analogs have been shown to reduce the time to fistula closure and the length of hospital stay, but do not increase the number of fistulas that close spontaneously.
Definition of the Fistula Tract
Once the patient is stabilized, definition of the fistula tract should be undertaken. The fistula should be assessed for point of origin, condition of adjacent bowel, presence of abscess, and any distal obstruction or bowel discontinuity. This can be accomplished with a range of radiological examinations: fistulagram, ultrasonography, magnetic resonance imaging (MRI), positive emission tomography (PET) scan, or computerized tomography (Arebi & Forbes, 2004; Schecter et al., 2009).
Nutritional Support
As previously discussed, adequate nutritional support is an essential component of effective management; it is critical to keep the patient in positive nitrogen balance to promote healing of the fistula tract. The route of nutritional support depends on the patient’s ability to ingest sufficient quantities, the location of the fistula tract, the absorptive capacity of the bowel mucosa, and the patient’s tolerance.
The use of total parenteral nutrition (TPN), accompanied by simultaneous “bowel rest,” has revolutionized the care of the fistula patient by allowing for the delivery of nutrition while simultaneously minimizing fistula effluent; this enhances patient management and the potential for spontaneous closure (Dubose & Lundy, 2010). On the negative side, the delivery of TPN through central venous catheters is associated with an appreciable rate of bacteremia and line sepsis. In one study conducted by Wong and colleagues (2004), positive blood cultures were obtained from 24.6% of 88 catheters utilized to deliver TPN to patients undergoing nonoperative management of enteric fistulas.
There is currently increased interest in the use of enteral nutrition for prevention and management of patients with ECFs, based on the role of enteral intake on the health and integrity of the intestinal mucosa; low-volume enteral intake can prevent translocation of bacteria; maintain the normal structural, immunologic, and hormonal integrity of the GI tract; and reduce cost relative to TPN (Dubose & Lundy, 2010). During a small retrospective study, Collier et al. (2007) noted that early postoperative initiation of enteral nutrition (≤4 days) resulted in a lower fistula formation rate than did nutritional approaches involving later initiation of enteral feedings (9% vs. 26%, respectively). Researchers also noted that the use of early enteral nutrition resulted in earlier primary abdominal closure and lower hospital charges.
CLINICAL PEARL
Nutritional support is a critical element of effective fistula management; the goals of nutritional management are to maintain positive nitrogen balance (usually through TPN), maintain the integrity of the intestinal mucosa (usually through low-volume enteral intake), and minimize fistula output (usually through reduced oral/enteral intake).
In selected patients, enteral nutrition may be used to maintain nutritional status while promoting fistula closure. It is now known that approximately 4 feet of healthy small intestine (in the adult) are needed to meet nutritional needs via the enteral route (Knechtges & Zimmermann, 2009). Therefore enteral nutrition may be feasible for the patient whose fistula is located in the most proximal or distal portion of a functional GI tract; if the fistula is located in the most proximal segment of the bowel, the enteral feeding must be administered distal to the fistula. Many types of enteral solutions are available, and a dietician should be consulted to recommend the most appropriate solution and administration procedure so that GI intolerance (e.g., diarrhea, abdominal distention) can be avoided.
Skin Protection and Containment
Establishing and maintaining skin protection and containment of the fistula effluent can be a challenging and yet rewarding experience. It is beneficial for WOC nurses managing the patient with an ECF to frequently remind themselves of the four general principles presented by Rolstad and Wong (2004).
1. Assess the pouching system and seal frequently; expect to make changes in the management system.
2. Build flexibility into the care plan.
3. Innovate, using the easiest, most practical approach first.
4. Recognize that care of the patient is frequently provided by inexperienced caregivers.
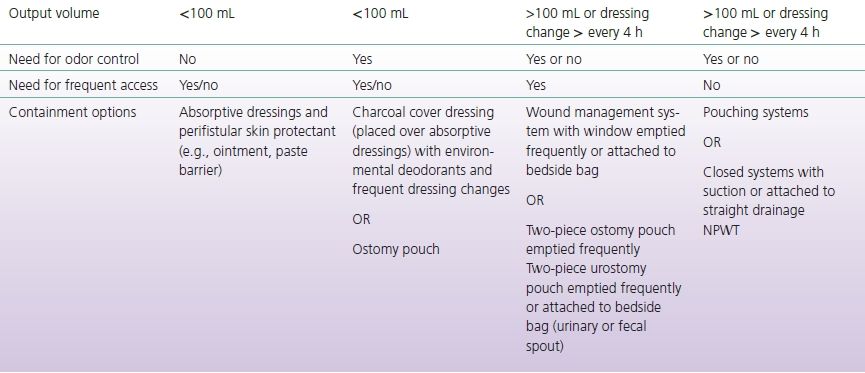
Skin protection and effluent containment should be initiated as soon as the fistula develops and is not contingent upon medical diagnosis. Goals for topical management of the ECF are listed in Box 17-1 (Bryant & Best, 2015). Methods and techniques for skin protection and containment will be described in the intervention section of the chapter and are presented in Tables 17-2 and 17-3.
TABLE 17-2 Fistula Products and their Indications
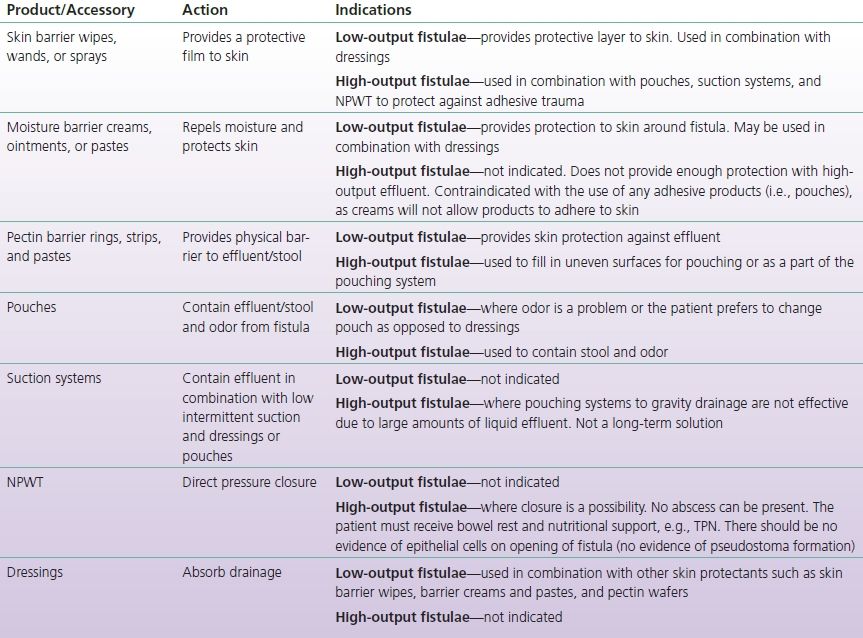
NPO, nothing by mouth; NPWT, negative pressure wound therapy; TPN, total parenteral nutrition.
TABLE 17-3 Fistula Containment Options Based on Output and Need for Access
BOX 17-1 Goals for Topical Management of the ECF
- Perifistular skin protection
- Containment of effluent
- Odor control
- Patient comfort
- Accurate measurement of effluent
- Patient mobility
- Ease of care
- Cost containment
CLINICAL PEARL
Skin protection and effluent containment should be initiated as soon as the fistula develops and is not contingent upon medical diagnosis nor the medical plan of care.
Fistula Management for the WOC Nurse
Methods and strategies for fistula management are guided by a thorough assessment; the parameters first described by Boarini and Bryant (1986) remain the standard for fistula assessment today (Bryant & Best, 2015; CAET, 2009).
Assessment
In addition to determining the type of fistula, assessment must include abdominal contours, fistula opening, effluent characteristics, and the condition of the perifistular skin. Each dressing or pouch change represents an opportunity to reevaluate the fistula and to modify the care plan accordingly. All assessments, reassessments, interventions, responses to interventions, and management and follow-up plans should be documented (see Box 17-2).
BOX 17-2 Documentation for the WOC Nurse
Focused Assessment
- Fistula source (see Table 17-1)
- Pain
- Fistula opening
- Location
- Length and width
- Height (retracted, skin level, protruding)
- Location
- Perifistular skin integrity
- Intact
- Impaired (erythema, maceration, candidiasis, denudement)
- Intact
- Abdominal contours and proximity of fistula to: scars, skin folds, bony prominences, drains, or ostomies
- Output/effluent
- Volume
- Consistency
- Color
- Odor
- Volume
- Containment system and frequency of changes
Interventions
- Emotional support
- Changes in containment method/procedure with rationale (if any change required)
- Education of patient/family
- Normal versus impaired skin
- Signs of infection
- Containment procedure
- Normal versus impaired skin
Evaluation
- Indicators of progress in closure (or impediments, such as pseudostoma formation)
- Effectiveness of containment system
- Wear time without leakage
- Perifistular skin intact or improved
- Odor control
- Effects on patient mobility
- Ease of care
- Wear time without leakage
- Patient/family response to interventions
- Patient satisfaction
- Comfort
- Level of activity
- Learning
- Patient satisfaction
Follow-up Plan
- Approximate day of next visit
- Instruction for staff between visits (including what to do if questions/concerns arise)
Progress toward/Impediments to Spontaneous Closure
A critical aspect of assessment is evaluation of progress toward and impediments to spontaneous closure (see Box 17-3). Progress in closure is evidenced by reduced output through the fistula tract along with increased fecal output through the distal bowel (rectum or stoma); thus, fistula output should be monitored, as should the output from any stoma, and the patient with an intact distal bowel should be routinely queried regarding bowel movements. Any indicators of abscess formation (e.g., increasing abdominal tenderness, fever, or purulent drainage mixed with the fecal output) must be promptly reported so that intervention can be initiated. The nurse must also be alert to development of a stomatized fistula, also known as a pseudostoma or an epithelialized stoma; this occurs when the anterior wall of the bowel becomes adherent to the abdominal wall and the fistula tract undergoes mucosal eversion. The end result is a permanent opening into the bowel that must be closed surgically; thus, observation of a “stoma” in the wound bed requires prompt MD notification (see Fig. 17-2).
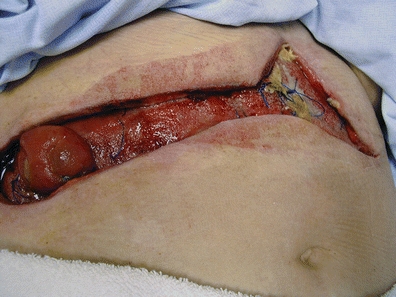
FIGURE 17-2. Pseudostoma in deep wound.
BOX 17-3 Factors that Prevent Spontaneous Closure
- Compromised distal suture line/anastomosis (i.e., tension on suture line, improper suturing technique, inadequate blood supply to anastomosis)
- Distal obstruction
- Foreign body in fistula tract or suture line
- Epithelium-lined tract contiguous with skin (pseudostoma)
- Presence of tumor or disease in site
- Previous irradiation to site
- Crohn’s disease
- Abscess
- Hematoma
Adapted from Bryant, R., & Best, M. (2015). Management of draining wounds and fistulas. In R. Bryant & D. Nix (Eds.), Acute and chronic wounds: Current management concepts (5th ed.). St. Louis, MO: Mosby. (In Print.)
CLINICAL PEARL
Assessment of the individual with a fistula must include the volume and characteristics of the output, the contours of the abdominal wall and fistula opening, and indicators of progress toward or impediments to spontaneous closure.
Abdominal Contours and Fistula Opening
The fistula opening and abdominal contours should be assessed while the patient is standing, sitting, and lying down if possible. If the pannus is large, more positions may be necessary to observe the changes in perifistular contours that occur with shifts in position of the pannus. Skin contours should be noted, and the abdomen should be inspected for irregular skin surfaces that are created by scars, creases, bony prominences, or other obstacles such as sutures/staples, incisions (dehisced or intact), or stomas. The location of the ECF should be identified, and visible openings should be measured (length and width in cm) and documented. The level at which the fistula empties in relation to the skin (or wound) surface is of critical importance. The fistula opening may be retracted (lower than skin level), level with the skin, above the level of the skin, or in a deep wound (see Fig. 17-2). If the fistula empties directly onto the skin and is level with the skin, a convex pouching system is usually required; in contrast, a fistula that has undergone mucosal maturation (pseudostoma formation) and that protrudes above the level of the skin may enable a better pouch seal. The opening might also contain a drain, as seen in Figure 17-1, for drainage of an abscess.
CLINICAL PEARL
A “pseudostoma” occurs when the anterior wall of the bowel becomes adherent to the abdominal wall, and the fistula tract undergoes mucosal eversion to create an epithelium-lined tract. Pseudostoma development requires MD notification because it means the fistula will have to be closed surgically.
Assessment of all these parameters will help determine the level of skin protection required, as well as the flexibility, size, and shape of adhesive barrier needed for effective protection of the perifistular skin and avoidance of any areas that could compromise the adhesion of the pouching system or cause discomfort to the patient.
Effluent Characteristics
Assessment of fistula effluent (source, volume, odor, consistency, composition) influences topical management selection by providing insight into the degree of risk for perifistular skin breakdown and odor as well as the type of pouch closure needed. For example, an odorous fistula producing effluent with semi-formed consistency is most likely originating from the left transverse or descending colon. Effluent from the transverse or descending colon will be less damaging to the skin than is output from the small intestine or stomach. (See Fig. 17-1 for illustration of skin damage related to small bowel drainage.) Thus, the primary goals of topical management for that patient would be containment of effluent and odor control.
ECF output volumes >100 mL over 24 hours usually require a pouch or suction or both. In contrast, the ECF with minimal output can often be managed with the application of a perifistular moisture barrier and an absorptive dressing. However, in the presence of odor, even the patient with a low-output fistula may prefer a containment pouch for odor control. It should be noted that odor may originate from numerous sources, including fecal drainage, exudate, necrotic or infected tissue, soiled dressings, and/or chemicals used during treatment.
CLINICAL PEARL
There are multiple options for containment of fistula effluent and perifistular skin protection; the best option for an individual patient is determined by the volume and characteristics of the output and the abdominal contours.
Consistency of effluent is particularly important to selection of a pouching system because it influences the type and number of skin barriers needed as well as the type of drainage outlet required to efficiently empty the pouch. For example, liquid effluent is much more corrosive than is thick effluent and is much more likely to result in premature erosion of the skin barrier; thus, liquid effluent requires the most durable skin barrier/protectant. Liquid effluent is easier to empty from a spout rather than clamp type closure.
Constant exposure of the epidermis to moisture, enzymes, extremes in pH, and mechanical trauma frequently leads to perifistular skin damage. Denudation of perifistular skin is a common complication in fistula patients and often is present when the patient is first seen with the fistula. Perifistular skin is also at risk for fungal infection as a consequence of moisture entrapment against the skin and antibiotic precipitated changes in the normal skin flora. Candidiasis is a common secondary complication and requires treatment with a topical antifungal agent. See Chapter 17 of the wound core curriculum for additional information regarding differential assessment and management of periwound MASD.
In addition to visual inspection, valuable information can be obtained from the patient and nursing staff. For example, patient reports of burning or stinging sensations around the fistula commonly indicate denudation or erosion of the epidermis, and the patient who requires frequent dressing or pouch changes is at risk for skin damage from mechanical injury in addition to damage from exposure to the effluent.
Interventions and Containment Strategies
Fistulas can be managed with skin protection and either dressings or containment devices (pouches, suction, negative pressure). There are a wide number of products available, and the appropriate selection is based on patient assessment. Principles of management warrant repetition: begin with the easiest approach based on assessment and sound rationale, reassess frequently and be flexible, and expect that needs will change. Interventions should be based on established principles and rationale, should include measures for skin protection and containment as well as patient and family support and education, and should be appropriately documented (see Box 17-2). Tables 17-2 and 17-3 present factors to consider when selecting containment methods.
CLINICAL PEARL
The recommended approach to determining the best system for effluent containment and skin protection is to begin with the simplest approach and modify as needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Etiologic Factors
Etiologic Factors Medical Management
Medical Management Surgical Closure
Surgical Closure Conclusions
Conclusions