CHAPTER 61 Clinical applications of the female sex hormones fall into two major categories: contraceptive and noncontraceptive applications. In this chapter, we focus on noncontraceptive uses. Contraception is discussed in Chapter 62. The principal noncontraceptive application of estrogens and progestins is menopausal hormone therapy (HT): estrogens are given to manage hot flushes and other menopausal symptoms; progestins are given to oppose estrogen-mediated stimulation of the endometrium. Because much of the clinical pharmacology of the estrogens and progestins is related to their actions during the menstrual cycle, understanding the menstrual cycle is central to understanding these hormones. Accordingly, we begin by reviewing the menstrual cycle. The anatomic and hormonal changes that take place during the cycle are summarized in Figure 61–1. As indicated, the first half of the cycle (days 1 through 14) is called the follicular phase, and the second half is called the luteal phase. One full cycle typically takes 28 days. The uterine changes that occur during the cycle are brought about under the influence of estrogens and progesterone produced by the ovaries. During the first half of the cycle, estrogens are secreted by the maturing ovarian follicles. As suggested by Figure 61–1, these estrogens act on the uterus to cause proliferation of the endometrium. At midcycle, one of the ovarian follicles ruptures and then evolves into a corpus luteum. For most of the second half of the cycle, estrogens and progesterone are produced by the newly formed corpus luteum. These hormones maintain the endometrium in its hypertrophied state. At the end of the cycle, the corpus luteum atrophies, causing production of estrogens and progesterone to decline. In response to the diminished supply of ovarian hormones, the endometrium breaks down. Two anterior pituitary hormones—follicle-stimulating hormone (FSH) and luteinizing hormone (LH)—play central roles in regulating the menstrual cycle. During the first half of the cycle, FSH acts on the developing ovarian follicles, causing them to mature and secrete estrogens. The resultant rise in estrogen levels exerts a negative feedback influence on the pituitary, thereby suppressing further FSH release. At midcycle, LH levels rise abruptly (see Fig. 61–1). This LH surge causes the dominant follicle to swell rapidly, burst, and release its ovum. Following ovulation, the ruptured follicle becomes a corpus luteum and, under the influence of LH, begins to secrete progesterone. After menopause, more bone is resorbed than is deposited, and hence bone mass declines. Why does this occur? Because the braking influence of estrogen on bone resorption is gone. Although estrogen therapy can help preserve bone mass, other drugs—raloxifene, bisphosphonates, calcitonin, and teriparatide—are safer, and hence are preferred (see Chapter 75). Does estrogen increase the risk of breast cancer? Yes—but primarily in postmenopausal women who are using estrogen combined with a progestin. In younger women, and in women using estrogen without a progestin, the risk, if any, is very low. Results of the Women’s Health Initiative (WHI) indicate that treatment of postmenopausal women with estrogen plus a progestin produces a small increase in the risk of breast cancer—but treatment with estrogen alone carries little or no risk (see below under Menopausal Hormone Therapy). In contrast, the Women’s Contraceptive and Reproductive Experience study has shown that, for most younger women, use of estrogen plus a progestin for contraception does not increase breast cancer risk (see Chapter 62). How can we explain these contradictory results? The best explanation is that risk is a function of age—being relatively high in older women and very low in younger women. In either population—old or young—the risk of breast cancer from estrogen alone has not been firmly established. Of note, after the results of the WHI were published, there was a dramatic decline in postmenopausal estrogen use, followed by a significant decline in the incidence of breast cancer. This observation reinforces the conclusion that postmenopausal hormone therapy increases breast cancer risk. In postmenopausal women, estrogen, used either alone or combined with a progestin, increases the risk of venous thromboembolism (VTE) and stroke. In addition, estrogen alone increases the risk of coronary heart disease and myocardial infarction, but only in women over age 60. The risk of VTE is greatest during the first years of treatment, and is greater with oral dosing than with transdermal or intravaginal dosing. These effects are discussed further under Menopausal Hormone Therapy. In premenopausal women, use of combination estrogen/progestin oral contraceptives increases the risk of VTE. Although the risk is dose dependent, even low-dose formulations cause an estimated 15 to 30 cases per 100,000 woman-years of use. The cardiovascular risk associated with oral contraceptives is discussed further in Chapter 62. Tamoxifen was the first SERM to be widely used. By blocking estrogen receptors, tamoxifen (and its active metabolite, endoxifen) can inhibit cell growth in the breast. As a result, the drug is used extensively to prevent and treat breast cancer. Unfortunately, blockade of estrogen receptors also produces hot flushes. By activating estrogen receptors, tamoxifen protects against osteoporosis and has a favorable effect on serum lipids. However, receptor activation also increases the risk of endometrial cancer and thromboembolism. The pharmacology of tamoxifen and toremifene (a close relative of tamoxifen) is discussed at length in Chapter 103. Raloxifene is very similar to tamoxifen. The principal difference is that raloxifene does not activate estrogen receptors in the endometrium, and hence does not pose a risk of uterine cancer. Like tamoxifen, raloxifene protects against breast cancer and osteoporosis, promotes thromboembolism, and induces hot flushes. Raloxifene is approved only for prevention and treatment of osteoporosis, and for prevention of breast cancer in high-risk women. Raloxifene is discussed at length in Chapter 75.
Estrogens and progestins: basic pharmacology and noncontraceptive applications
The menstrual cycle
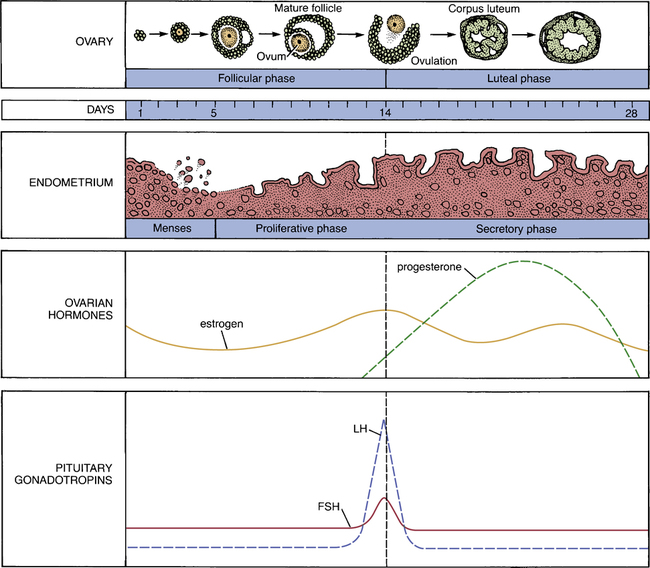
 The menstrual cycle: anatomic and hormonal changes.
The menstrual cycle: anatomic and hormonal changes.
(FSH = follicle-stimulating hormone, LH = luteinizing hormone.)
The roles of estrogens and progesterone.
The role of pituitary hormones.
Estrogens
Biosynthesis and elimination
Physiologic and pharmacologic effects
Metabolic actions
Bone.
Clinical pharmacology
Adverse effects
Breast cancer.
Cardiovascular events.
Selective estrogen receptor modulators (SERMs)



