Chapter 17. Endocrine system V
Hormones and reproduction
At the end of this chapter, the reader should be able to:
• describe the main phases of the menstrual cycle and the roles of the various hormones
• list the therapeutic uses of the estrogens and the different types of oral contraceptive pill and a few examples of each
• explain the mechanisms of action and administration of the different types of oral contraceptives
• describe the beneficial and reported adverse effects and the controversies surrounding the use of oral contraceptives
• describe the symptoms of menopause, the types of preparations used for hormone replacement therapy (HRT) and how they are used
• discuss the obstetric use and risks associated with the use of prostaglandins, ergometrine and oxytocin
• list the drugs now used for pregnancy termination and describe the treatments for menorrhagia and dysmenorrhoea
• describe the premenstrual syndrome (PMS) and its symptoms
The female sex hormones
It is important to understand the hormonal background of the normal menstrual cycle and of pregnancy before considering the individual hormones.
The menstrual cycle
The primary purpose of the menstrual cycle is to grow an ovarian follicle and its enclosed ovum to a point when the ovum is released from the follicle and is ready to be fertilized, while at the same time preparing the female reproductive tract for the entry of the male sperm and for the implantation of the fertilized egg into the inner wall or endometrium. The critical event is the explosive rupture of the follicle at mid-cycle and the release of the ovum into the fallopian tubes, where the egg will be fertilized by one of the spermatozoa if these are present. The fertilized egg will travel down to the uterus, dividing as it goes, where it will implant itself in the endometrium of the uterus. If the ovum is not fertilized and implantation does not occur, progesterone secretion stops and this may be one of the triggers for menstruation. The entire process is superbly orchestrated by the combined and synchronized actions of hormones from the brain, the anterior pituitary and from the ovary itself.
Hormonal control of the menstrual cycle
This is facilitated by:
• a hypothalamic hormone: gonadotrophin-releasing hormone (GnRH)
• anterior pituitary gonadotrophins: follicle-stimulating hormone (FSH) and luteinizing hormone (LH)
• ovarian sex hormones: estradiol-17β and progesterone.
The menstrual cycle and ovulation are made possible through the operation of feedback systems involving the hypothalamus, anterior pituitary and the sex hormones estradiol-17β and progesterone, which are released by the ovarian follicle and corpus luteum, respectively. The feedback systems are similar in principle to those that govern the secretion of thyroid hormone and cortisol, in that hormones act on the pituitary and the hypothalamus to suppress the release of hormones that cause sex hormone release from the gonads. They differ from systems that control, for example, thyroxine release, in that there are also positive feedback effects at the level of the pituitary and the hypothalamus in operation to cause more release of sex hormones at critical times of the menstrual cycle. The menstrual cycle has three main components: the proliferative phase, the luteal phase and menstruation.
Proliferative phase of the cycle
In primates, including humans, the hypothalamus synthesizes and once every 60–90 minutes releases into the pituitary portal system a peptide called gonadotrophin-releasing hormone or GnRH (see also p. 171). This intermittent release of GnRH is called a ‘pulsatile’ release. GnRH acts on anterior pituitary cells called gonadotrophs, causing them to release FSH into the general circulation. In the ovary, FSH promotes the growth of the follicles. Each follicle contains an ovum, and, for some reason, one follicle (and sometimes two follicles) develops faster than the others and it becomes the Graafian follicle, and the other follicles degenerate. The Graafian follicle synthesizes the powerful estrogenic hormone estradiol, which is released into the general circulation. Another two estrogenic hormones released into the circulation are estrone and estriol.
As the Graafian follicle matures, it releases more and more estradiol-17β into the circulation. Estradiol travels throughout the body, where it works busily to prepare the reproductive tract for the coming ovulation:
• In the uterus it causes the regeneration of the endometrium or inner lining of the uterus.
• In the anterior pituitary, though a negative feedback effect, it prevents GnRH from causing a release of LH from gonadotroph cells, thus preventing LH from reaching the follicle before the follicle is ready to be ruptured.
• Estradiol works to prepare the gonadotrophs of the anterior pituitary so that they become more sensitive to hypothalamic GnRH.
• Another important job of estradiol is to cause a large increase in the concentration of progesterone receptors in the endometrium, anterior pituitary and hypothalamus. This is done to prepare these tissues for the rise in progesterone secretion that will occur after ovulation. This period before ovulation is called the follicular or proliferative phase of the cycle.
Ovulation
Ovulation occurs about halfway through the normal 28-day menstrual cycle due to a mid-cycle explosive discharge of LH from the anterior pituitary. This occurs because estradiol has made the anterior pituitary gonadotrophs exquisitely sensitive to hypothalamic GnRH. In addition, for some unknown reason, the powerful negative feedback effect of estradiol on LH release is overcome. This LH surge causes the rapid swelling and rupture of the follicle and the egg is released. The ruptured follicle now becomes the corpus luteum (Latin for yellow body). Knowledge of these events during the menstrual cycle has made it possible to advise on how to optimize the chances of falling pregnant.
The luteal phase
The part of the cycle following ovulation is called the luteal phase. The corpus luteum produces the hormone progesterone, which has a number of critical actions:
• Progesterone causes further thickening of the endometrium through the build-up of glands and laying down of glycogen; this is called a secretory endometrium.
• Progesterone exerts a negative feedback effect on the anterior pituitary, suppressing the release of LH.
• The hormone, perhaps by an action on the hypothalamus, causes an increase in body temperature of about 0.5°C, its so-called thermogenic effect.
• Progesterone is responsible for some water and salt retention and is mildly anabolic.
• Progesterone ensures that any incoming sperm will find a hostile environment by making the cervical mucus more viscid and less alkaline.
The endometrium now passes into what is called its secretory phase. If implantation of the fertilized ovum does not occur, the corpus luteum regresses and the superficial part of the endometrium breaks down and is discharged as the menstrual flow (Fig. 17.1).
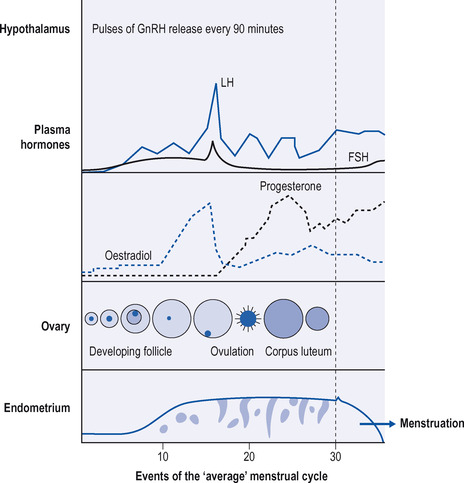 |
| Figure 17.1 Events of the ‘average’ menstrual cycle. (Reproduced with permission from: Greenstein B. Endocrinology at a glance. Cambridge, MA: Blackwell Sciences, 1994: p. 49) |
Conception
It is not always easy for couples to conceive, and the information available about the menstrual cycle enables the nurse to give advice. It is most important to ascertain when ovulation occurs. This can be done by taking the temperature throughout the cycle. As stated above, the temperature rises after ovulation by about 0.5°C due to the thermogenic action of progesterone. Couples should have intercourse anywhere from 4–5 days before ovulation to 24 hours afterwards, with the best chances of conception if intercourse is within the time window of 24 hours before or after ovulation. The figures arrived at above are based mainly on the fact that spermatozoa live up to 72 hours after entry into the female reproductive tract. The most fertile days for a woman with the 28-day cycle are days 12–18, with ovulation occurring on day 14.
Pregnancy
If a fertilized ovum is implanted in the uterus, the corpus luteum does not immediately regress, but continues to produce its hormones. This function is eventually taken over by the placenta. Throughout pregnancy, large quantities of progesterone and estrogens are produced by the placenta, and can be recovered from the urine. The human placenta produces a gonadotrophic hormone called chorionic gonadotrophin during the early months of pregnancy and its presence in the urine forms the basis of various tests for pregnancy. The placenta also produces large amounts of the estrogenic hormone estriol, and levels of estriol are used to monitor the growth of the fetus. The most important clinical monitoring aid for fetal development, however, is through the use of ultrasound.
Just before parturition, the production of progesterone ceases and this may be concerned with the start of labour.
The reader will appreciate that knowledge of the various negative feedback mechanisms governing LH and FSH release can and has provided the rationale for the design of the oral contraceptives (see below).
The male sex hormones
The hypothalamus of the postpubertal male puts out its regular pulsatile dose of GnRH and in response the anterior pituitary puts out FSH and LH. FSH promotes the development of the spermatozoa and LH promotes the production of testosterone by the interstitial Leydig cells of the testis. Testosterone exerts androgenic and anabolic effects (see below) and also has a negative feedback effect on LH secretion from the anterior pituitary, thus regulating its own production in the testis.
Clinical use of gnrh analogues
GnRH is a strange hormone. It is a peptide, and if it is administered to the pituitary in pulsatile fashion, it ensures normal anterior pituitary function and continued fertility. If GnRH for some reason is not produced, infertility results. If, on the other hand, the pituitary receives a continuous exposure to GnRH, it actually shuts down gonadotrophin production by the anterior pituitary cells. These phenomena have been exploited either to restore fertility or to prevent sex hormone production by the gonads. GnRH has been prepared synthetically and more stable and powerful analogues introduced.
Synthetic GnRH and GnRH analogues
These comprise:
• gonadorelin, which is synthetic GnRH
• buserelin
• goserelin
• leuprorelin
• nafarelin, which is about 200 times more powerful than GnRH.
The last four drugs are synthetic analogues of gonadorelin. An analogue of a hormone is a synthetic compound with a (usually) slightly modified chemical structure but the same biological actions. The analogues mentioned above all act on the GnRH receptors on anterior pituitary cells and are therefore also called agonists.
Therapeutic uses
Gonadorelin
Gonadorelin is used to induce ovulation in some cases of infertility. It is given as a pulsed injection every 90 minutes using a miniaturized pump.
GnRH analogues
GnRH analogues are usually given as subcutaneous, long-acting implants. They initially increase the release of gonadotrophins, followed by a falling off of gonadotrophin secretion due to pituitary desensitization. This results in decreased activity of the male and female gonads and reduced secretion of the sex hormones. GnRH analogues are used for the treatment of severe cases of endometriosis and carcinoma of the prostate. The aim is to shut down the production of the sex hormones, which aggravate both conditions. They are also used together with iron supplements to treat anaemia due to uterine fibroids.
When these GnRH analogues were first introduced and implanted into men with carcinoma of the prostate, the initial stimulus to gonadotrophin release caused a sometimes-fatal acceleration of the carcinoma due to increased testosterone production. To counteract this, patients are also treated with a drug such as cyproterone acetate (see below), which blocks the action of androgens on their receptors.
Clinical use of gonadotrophins and antagonists
These comprise:
• FSH
• human chorionic gonadotrophin (HCG)
• human menopausal gonadotrophin (HMG: FSH+HCG)
• clomifene
• danazol
• gestrinone.
These hormones and synthetic compounds are used therapeutically and will be considered in detail (Fig. 17.2).
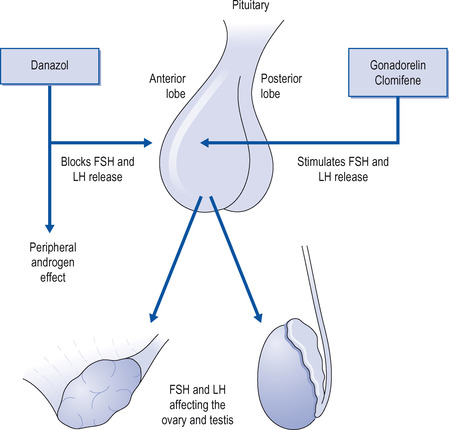 |
| Figure 17.2 Drugs modifying the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and thus modifying gonadal activity. |
FSH and LH
FSH is available as urofollitropin and as follitropin alpha and beta. It is extracted from the urine of postmenopausal women. In the female it causes ripening of the ovarian follicles and the production of estrogen, and in the male it is necessary for the production of spermatozoa. It is given by injection. Pituitary LH is not used, but its actions are available as HCG. It is extracted from the urine of pregnant women. In the female it produces the corpus luteum and in the male it stimulates the interstitial cells of the testis to produce androgens. It is given by injection.
In female infertility, FSH and HCG are given by injection to induce normal ovarian function. FSH is given first to produce an ovarian follicle, followed by HCG to induce ovulation, or they may be given together as HMG. They will only be successful if infertility is due to lack of normally secreted gonadotrophins and not if there is primary ovarian failure.
Clomifene and cyclofenil
Clomifene is a synthetic compound that blocks the action of estradiol on its receptors (see more below). This releases the anterior pituitary from estradiol’s negative feedback effects, and large amounts of LH and FSH are released. Clomifene stimulates increased secretion of gonadotrophins and is used in the treatment of infertility due to failure of ovulation. It is given daily for 5 days early in the menstrual cycle. It may be so successful that it results in twins, but multiple pregnancies can be avoided by careful dosing. Adverse effects include flushing, headaches, nausea, weight gain and visual disturbances.
Danazol and gestrinone
Danazol and gestrinone inhibit both GnRH and gonadotrophin release and are used to treat endometriosis and various benign breast disorders: for example, cyclical breast pain. This is due to swelling and tenderness of the breasts, which occurs during the second half of the menstrual cycle and is associated with the corpus luteum. If the symptoms are severe, danazol is effective in prevention, but its adverse effects are troublesome. The adverse effects reflect the fact that these compounds are androgen derivatives and cause abnormal hair growth, greasy skin, acne, fluid retention, weight gain and nausea.
Patients may ask about gamolenic acid, which is extracted from evening primrose oil; this may decrease the sensitivity of the breasts to hormones and may thus also be effective, but relief is delayed for about 3 months.
• GnRH analogues are used to treat endometriosis and prostatic carcinoma
• When GnRH is started in patients with prostate carcinoma, they should take an androgen receptor blocker as well
• FSH and HCG (or HMG) will be successful in treating infertility only if infertility is caused by a lack of normally secreted gonadotrophins
• Clomifene is used to treat infertility due to failure of ovulation and can cause multiple births
• Danazol and gestrinone are used to treat cyclical breast pain, although danazol has unpleasant side-effects
The estrogens
As mentioned above, estradiol is the main female sex hormone and the most potent. Estrone is also a female sex hormone but is shorter-acting. Estriol is produced in large amounts during pregnancy, but its function is obscure.
Therapeutic use of estrogens
The estrogens comprise natural estrogens and synthetic estrogens (Table 17.1). Estradiol-17β is the principal estrogen secreted by the ovary, but there are a number of estrogens that are used therapeutically. Estrogens are used for:
• ORAL CONTRACEPTION
• hormone replacement therapy
• (rarely) controlling cancer of the prostate and breast
• atrophic vaginitis (as a cream).
| *Used topically in the vagina. | |
| Natural estrogens | Synthetic estrogens |
|---|---|
| Estradiol-17β | Ethinylestradiol |
| Estrone | Mestranol |
| Estriol | Dienestrol* |
Oral contraception
There are two main types of oral contraceptive pill (the Pill):
• the COMBINED ORAL CONTRACEPTIVE PILL
• the progestogen-only pill.
Combined oral contraceptive pill
The combined oral contraceptive pill is very widely used and is the most effective method of preventing conception. It is a combination of an estrogen and a progestogen and acts in several ways. The estrogen used is ethinylestradiol or mestranol (a few). The progestogen used is desogestrel, gestodene, etynodiol, levonorgestrel or norethisterone.
Mechanism of action
• The estrogen inhibits the release of FSH by a negative feedback effect, thus inhibiting follicular development.
• The progestogen inhibits the release of LH, so that ovulation cannot occur. Together, the two chemicals render the endometrium hostile to implantation.
• Both chemicals may upset the coordinated contractions of the fallopian tubes, uterus and cervix.
Usually, the composition of the Pill is unaltered throughout the full monthly course, but there are a few preparations in which pills of varying composition are given sequentially: namely, the biphasic and triphasic preparations. Of the many preparations now available, the most effective and widely used are those in which both an estrogen and a progestogen are given throughout the course, with a failure rate of less than 0.5 per 100 women-years. Table 17.2 shows the estrogen and progestogen content of some of the preparations in use.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
