Chapter 14. Endocrine system II. Hormones and metabolism
Thyroid, parathyroid glands, calcitonin and osteoporosis
At the end of this chapter, the reader should be able to:
• describe the anatomical location of the thyroid gland
• explain how thyroid hormones are synthesized, stored in the thyroid and how they are released
• give the causes, consequences and treatments of hypothyroidism
• describe the symptoms and types of hyperthyroidism
• explain the surgical treatment of thyrotoxicosis
• list the drugs used to treat thyrotoxicosis
• describe the actions of parathyroid hormone (PTH)
• explain how calcium absorption is facilitated and how osteoporosis is treated
The thyroid gland and thyroid hormones
Introduction
The thyroid consists of two lobes connected by an isthmus and is situated in the neck, in front of the trachea (Fig. 14.1). Functionally, the thyroid gland is made up of follicles (sometimes called acini), which consist of a single layer of epithelial cells enclosing the follicular lumen. The lumen is packed with a colloidal protein called thyroglobulin, which is the storage vehicle for the thyroid hormones (see below). Circulating iodine is picked up by the epithelial cells of the thyroid gland and incorporated to form two hormones, thyroxine (T4) and triiodothyronine (T3). These are stored in the thyroid as a protein, thyroglobulin, which is called colloid (Fig. 14.2). With appropriate stimulation, notably through the action of thyroid-stimulating hormone (TSH; thyrotrophin), both T4 and T3 are released into the bloodstream (Fig. 14.3; see also the previous chapter, p. 174).
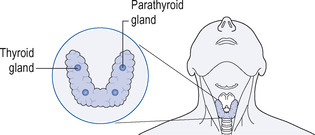 |
| Figure 14.1 The thyroid and parathyroid glands. |
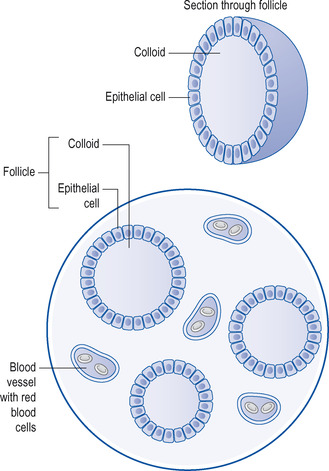 |
| Figure 14.2 Microscopic view of thyroid gland and follicle. |
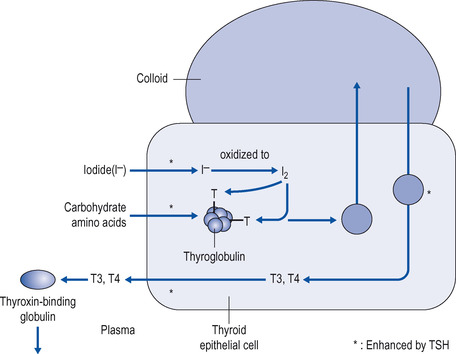 |
| Figure 14.3 Production, storage and release of thyroid hormones. Iodide is taken up into the thyroid cell and oxidized to iodine, which is incorporated into tyrosine, on thyroglobulin. The protein is stored as colloid, until thyroid-stimulating hormone (TSH) causes the cell to break down thyroglobulin and release triiodothyronine (T3) and throxine (T4), which are released into the circulation. CHO=carbohydrate, T=tyrosine residue on thyroglobulin, I 2=iodine. Starred reactions are promoted by TSH. |
On reaching the tissues, T4 is converted to T3, which is the more active hormone. T3 exerts its effects on the cell by binding both to receptors inside the cell in a manner similar to that of the steroid hormones, resulting in changes in protein synthesis, and to receptors on the cell surface (see below). The effect of thyroid hormones is to increase tissue metabolism and thus to raise the basal metabolic rate. They are also important in promoting growth (see below). In healthy people the release of thyroid hormone is nicely adjusted to maintain the metabolic rate at a satisfactory level. When thyroid hormone levels are normal, the patient is described as euthyroid. When they are clinically elevated, the patient is hyperthyroid. When they are clinically depressed, the patient is hypothyroid.
Production and release of thyroid hormones
Uptake of iodide into the thyroid
When iodide ions enter the circulation they are rapidly and powerfully taken up into the thyroid by an energy-dependent mechanism that can pump iodide into the thyroid against a concentration gradient of anything up to 50:1. In other words, if there is 50 times more iodide in the thyroid than in the plasma, it will still be taken into the thyroid gland.
Biosynthesis of T3 and T4
Once in the thyroid, iodide is oxidized to iodine and incorporated into tyrosine residues on the protein thyroglobulin such that both T3 and T4 are stored as part of the protein. The hormones are thus stored as part of a protein complex referred to as colloid. When circulating TSH from the anterior pituitary gland binds to its receptor on the thyroid cell, this triggers a response whereby droplets of the colloid are broken down in the cell and the hormones are released from colloid and secreted from the cell into the circulation. It is important here to give these processes in some detail, because drugs to treat thyrotoxicosis target some of the steps in the synthesis of thyroid hormones (see below). The process is summarized in Figure 14.3.
Plasma binding of thyroxine and thyroid function tests
Once in the circulation, a specific thyroxine-binding protein, thyroxine-binding globulin (TBG), takes up the thyroid hormones, binds them and they are carried to their sites of action. There is a dynamic equilibrium in the circulation between the bound and free forms of thyroid hormones. This is clinically important since it is only the free fraction of hormone that is available to the tissues, and free levels of thyroid hormones are measured in patients as well as total levels. The levels of TBG are also measured in some tests. As mentioned in the previous chapter, p. 174, the ability of the pituitary to release TSH in response to hypothalamic TSH-releasing hormone (TRH) is also used to diagnose forms of thyroid disease.
Action of thyroid hormones
When T4 reaches its site of action, it is converted to T3, which binds to receptors on the cell surface and increases the cell’s uptake of amino acids and glucose to increase metabolism. Inside the cell, T3 binds to intracellular receptors in the mitochondria and in the cell nucleus to generate energy and new proteins, respectively.
Abnormalities of thyroid function
These can be divided into:
• hypothyroidism (thyroid deficiency; myxoedema)
• hyperthyroidism (thyroid excess; thyrotoxicosis).
Hypothyroidism
Thyroid deficiency means a reduced availability to the body of thyroid hormone and can result from:
• Impairment of the TRH–TSH system of the brain and pituitary.
• Insufficient iodine in the diet; this is called simple or non-toxic goitre. Insufficient dietary iodine causes an increase in TSH secretion, which in turn causes thyroid growth, causing the goitre in the neck. Non-toxic goitre can also occur through the eating of certain plants, such as cassava root.
• Hashimoto’s disease, an immunological disorder, when the body reacts against the protein thyroglobulin, which is the mechanism for storing T3 and T4 in the thyroid gland, or to some other protein in the gland.
• Over-treatment with radioactive iodine, which is used to treat thyroid tumours (see below).
When thyroid deficiency is severe, it causes a condition called myxoedema. The term myxoedema means thickened skin and gives the disease its name. The symptoms of myxoedema are:
• mental impairment
• slow or slurred speech and deep, low voice
• bradycardia (slowed heart rate)
• lethargy
• sensitivity to cold
• coarse, dry skin.
Cretinism
The thyroid system is essential in the newborn infant for normal growth and development through the direct actions of T3 on the cells and through the influence of TSH on growth hormone release from the anterior lobe. If severe thyroid deficiency occurs in infants from birth and is left untreated for too long, it will give rise to cretinism, when development in the baby is stunted, causing dwarfism, mental retardation, and coarsened facial features and skin. It is also called congenital hypothyroidism.
Treatment of hypothyroidism
Hypothyroidism is treated either by increasing iodine in the diet and/or treatment with T3 or T4, both of which are available as oral tablets. Two preparations are effective in the treatment of thyroid deficiency: levothyroxine, which is the pure hormone synthetically prepared and used for long-term treatment, and liothyronine.
Levothyroxine
Levothyroxine sodium (T4; thyroxine sodium) tablets given orally are absorbed from the intestinal tract, but their full effects are not seen for about 10 days. If they are given to patients with cretinism or myxoedema, they will cause them to return to normal metabolic function.
It is important to start treating newborn infants with hypothyroidism as soon as possible because if they are left hypothyroid for too long, the change may be irreversible. In myxoedema it is very important to start with a small dose, otherwise the undue stimulating effect on the heart may cause untoward effects, including anginal pain. Early in treatment the patient should be kept warm, hypnotics should be avoided, and constipation, which is common, should be relieved.
Effects of overdosage:
Large doses will cause an excessive rise in metabolic rate and the symptoms of thyrotoxicosis, with loss of weight, tachycardia, nervousness and tremors. Although the dose can be monitored by the clinical response of the patient, it is preferable to measure the plasma T4 and TSH occasionally to ensure that the correct amount of hormone is being given.
Liothyronine
Liothyronine is the official name of triiodothyronine (T3). Its actions are similar to those of thyroxine, but are much more rapid in onset, the maximum effect being seen after 3 days. Liothyronine is not as useful as thyroxine in treating myxoedema, as the control of the disease is apt to be uneven, but it is useful if a rapid effect is required.
In both cretinism and myxoedema, it is usually necessary to continue treatment for the rest of the patient’s life.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
