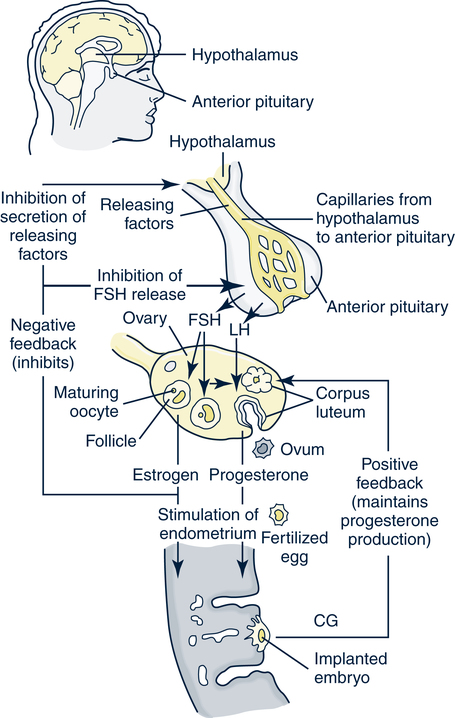
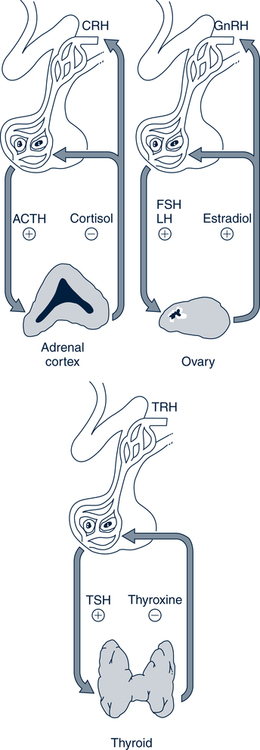
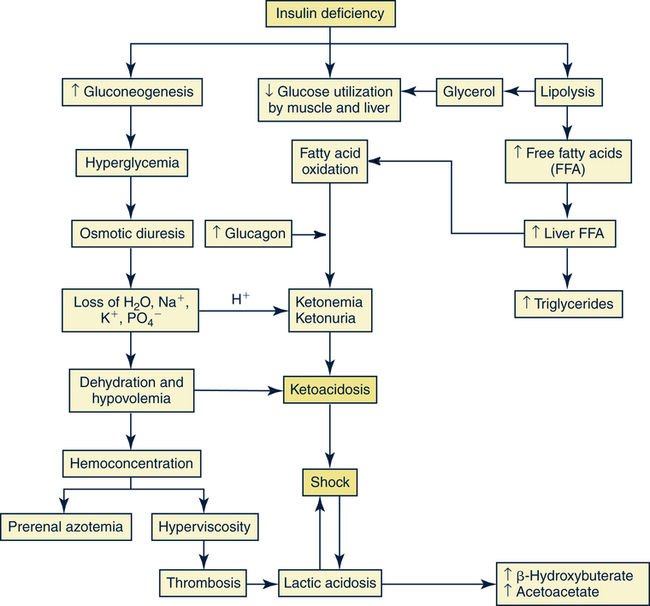
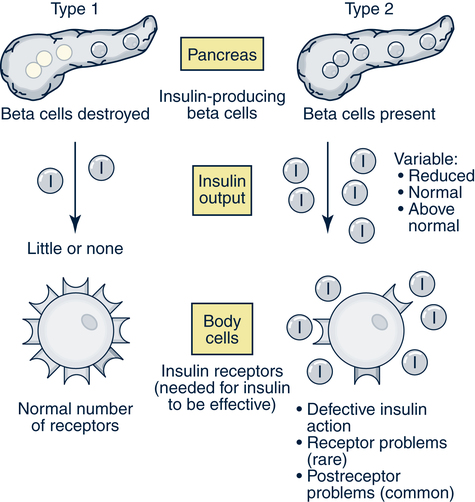
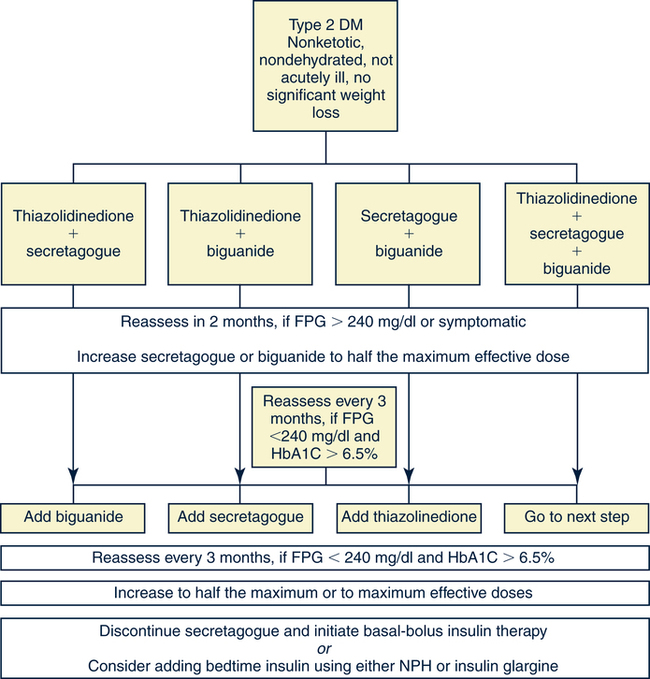
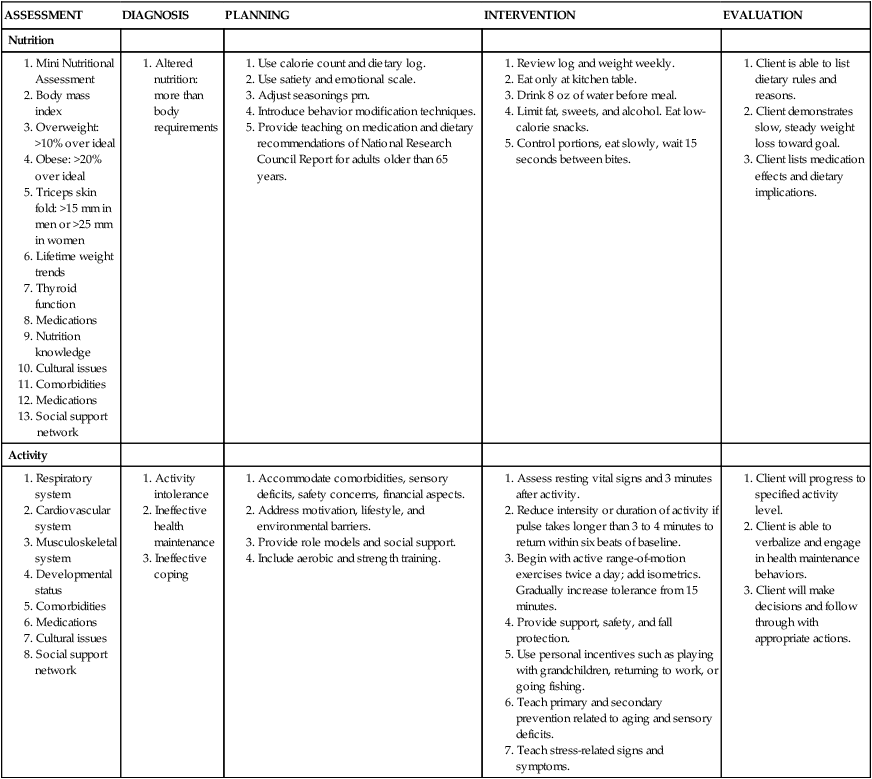
Catherine Hill, DNP(c), RN, GNP-BC On completion of this chapter, the reader will be able to: 1. Discuss the normal age-related physiologic changes that occur in the endocrine system. 2. Describe the major characteristics of common endocrine diseases in older adults: metabolic syndrome, type 2 diabetes mellitus, hyperthyroidism, hypothyroidism, osteoporosis, and sexual dysfunction. 3. Apply the nursing process in caring for an older adult with an endocrine disorder. Previously dominated by diabetes and thyroid disease, gerontologic endocrinology has recently been redefining itself through the use of innovative insights developed from the mapping of the human genome (Kenyon, 2005). Our knowledge of aging endocrine physiology and genetic influences (Garinis, van der Horst, Vijg, & Hoeijmakers, 2008) has begun to grow at a very fast pace. New animal models (Toivonen & Partridge, 2009) and genomic endocrine-related trait studies (Hwang, Yang, Meigs, Pearce & Fox, 2007) have lead to a robust subspecialty often referred to as the endocrinology of aging (Bellino, 2006). Andropause, circadian dysrythmias, dehydroepiandrosterone (DHEA) replacement, erectile dysfunction, glucagon-like peptide 1 replacement, male osteoporosis, menopause, metabolic syndrome, and metabolic presbycusis have joined the traditional topics of diabetes and thyroid disease in the newly emerged subspecialty. Endocrinology’s new “ensemble view of neuroendocrine aging” is now discussed in terms of decreased estrogen production in women (menopause), decreased testosterone production in men (andropause), decreased adrenal function (adrenopause), and decreased growth hormone–insulin-like growth factor (somatopause) (Bellino, 2006). Endocrinologic aging involves increased molecular disorderliness of the endocrine regulatory mechanisms that results in reduced vitality of the overall person. This molecular dysregulation of neurohormones from or with the central nervous system (CNS) is one of the earliest measurable characteristics of endocrine aging. Many believe natural endocrine aging is not a disease to be cured; others believe that our knowledge can provide important “antiaging” therapies that will benefit humans as a whole (Gardner & Shoback, 2007). Composed of ductless glands (Fig. 25–1, A), which secrete 40 major hormones (Copstead & Banasik, 2005) that control numerous processes throughout the body (Table 25–1), the endocrine system uses a delicate balance of chemical messengers in the bloodstream to excite and regulate mood, growth, organ function, metabolism, and sexual activity (Gardner & Shoback, 2007). Dependent on a complex interplay of factors, many hormones are secreted in a cyclic pattern of minutes, hours, days, or months. Feedback control processes (Fig. 25–1, B) of these intricate gland–hormone–organ–tissue systems depend on secretion and degradation of hormones classified by chemical structure and cell receptor type (Copstead & Banasik, 2005). TABLE 25–1 Data from Beers MH, Berkow R, editors: Merck manual of geriatrics, 2000–2005, Merck. Retrieved May 30, 2009, from http://www.merck.com/mrkshared/mm_geriatrics/home.jsp.; Copstead LE, Banasik JK: Pathophysiology, ed 3, St Louis, 2005, Elsevier. Apoptosis (cell death) is a theme that has dominated cellular research on the physiology of aging and some age-related diseases since 1972 (Mobbs & Hof, 2009). Additionally, three basic categories are used to classify endocrine pathology: hyposecretion, hypersecretion, and hyporesponsiveness; the system is elaborate and increases in complexity with the aging process (Table 25–2). In addition, clinical manifestations in the older person may be altered by disease processes in other body systems such as in the syndrome of inappropriate antidiuretic hormone secretion, which occurs with many types of tumors. Therefore, this chapter discusses the typical aging changes of menopause, andropause, adrenopause, and somatopause physiology without discussion of other potential superimposed pathophysiologic states. TABLE 25–2 AGING CHANGES IN THE ENDOCRINE SYSTEM Older men and women experience a decline in the biosynthesis and balance of their sex hormones from the cholesterol precursor as they age (Mobbs & Hof, 2009). In both genders, the hypothalamus–anterior pituitary–testes/ovary system declines, although the timing is gender specific. Both genders may experience hot flashes, night sweats, depression, and sexual dysfunction in response to age-related declines in androgen or estrogen. In contrast to the previous gender similarities in symptoms, laboratory values to determine the endocrine decline are unique to each sex: luteinizing hormone (LH) and testosterone are of primary importance in men, whereas follicle-stimulating hormone (FSH) and estrogen are of primary importance in women. Hormone replacement therapy, in both genders, is a hotly debated topic among intelligent providers because risks and benefits are unique to each patient. Ongoing debate over whether aging is a disease contributes to the controversy. Those who advocate testosterone replacement cite the benefits of improvements in relation to bone density, libido, muscle mass, strength, visuospatial skills, depression, fatigue, hot flashes, irritability, mood, and sleep (Kapoor, Goodwin, Channer, & Jones, 2006). Testosterone replacement in andropause is complicated by adverse lipid effects, the risk of promoting prostate cancer, worsening of sleep apnea, potential hepatotoxicity, increased aggressive behavior, and the risk of erythrocytosis. Menopausal and postmenopausal hormone replacement (HR) practices continue to change based on larger, more rigorous research studies The presence or absence of a uterus and ovaries guides clinicians on the types of hormones used in perimenopausal women (Ylikangas, Sintonen, & Heikkinen, 2005). The 2002 Women’s Health Initiative findings of increased breast cancer, heart disease, stroke, and blood clots from perimenopausal HR have recently been confirmed (Vickers et al, 2007) and joined by evidence of improved metabolic syndrome indices (Salpeter et al, 2006) and bone health with phytoestrogen HR (Cassidy et al, 2006), brain health (Erickson et al, 2007), and weight control (Foidart & Faustman, 2007). While many clinicians continue to prescribe perimenopausal HR, most agree that long-term HR is no longer clinically justifiable (Tormey, Malone, McDermott, et al 2006). Weighing approximately 4 g, the adrenal glands sit on top of the kidneys and are composed of the adrenal medulla and cortex. A total loss of adrenocortical function causes death within days; however, age-related decreases in mineralocorticoids, glucocorticoids, and androgenic hormones manifest changes in body composition, skeletal mass, muscle strength, body weight, and metabolism (Mobbs & Hof, 2009). Age-related decreases in DHEA and norepinephrine can produce fluid and electrolyte imbalances and changes in glucose, protein, and fat metabolism. The decline of DHEA with age parallels that of growth hormone, so by age 65 the human body makes only 10% to 20% of what it did at age 20 (Szkrobka, Krysiak, & Okopieri, 2008). These declines closely parallel declines in the growth hormone–insulin-like growth factor 1 axis, a process now referred to as somatopause. Somatopause is often spoken of from a neuroendocrine point of view because certain neurons in the hypothalamus secrete hormones (neurosecretion). Somatopause focuses on the neuron–hypothalamus–pituitary axis and the failure of central nervous system (CNS) integration of the endocrine and nervous systems, which causes peripheral endocrine gland insufficiency contributing to a disrupted feedback axis in aging (Martin, 2007). Specifically, somatotropin secretion from the hypothalamus– pituitary axis influences many age-related changes in nutrition, metabolism, body temperature, and circadian rhythms, circulation, salt/water balance, growth, and reproduction. Current antiaging researchers who believe “you’re as young as your oldest part” (Rothenberg, 2006) have focused on various secretagogue compounds that stimulate pulsatile growth hormone secretion and increase insulin-like growth factor 1 in the older adult to levels approximating those found in young adults. Metabolic syndrome is a common multifactorial syndrome of aging (the incidence is 26 per 1000 person-years) (Suzuki et al, 2008), which varies among racial and ethnic groups and is strongly associated with abdominal obesity in America (Grundy et al, 2005). Suspected endocrine influences on the syndrome include corticosteroid axis derangement, polycystic ovary syndrome, and dysglycemia. Recent epidemiologic research has identified, defined, and measured the metabolic syndrome as a significant antecedent to American illness trends in diabetes and heart disease (Grundy et al, 2005). Insulin resistance causes increased production of inflammatory cytokines correlating with the development of type 2 diabetes mellitus and atherosclerotic vascular disease. The primary risk factors for the syndrome are abdominal obesity, insulin resistance, physical inactivity, and hormonal imbalance (Grundy et al, 2005). Additionally, some evidence exists for genetic influences through a variety of gene polymorphisms (Laakso, 2004). The reduction of risk factors for diabetes and atherosclerotic disease are the primary therapeutic objectives in metabolic syndrome (Grundy et al, 2005). The therapeutic lifestyles changes (TLCs) that will improve all metabolic risk factors are detailed in (Box 25–1). Nutritional management for metabolic syndrome should include meticulous attention to the amounts of low-saturated fats, trans-fat, cholesterol, and simple sugars. A slow, modest weight loss of 7% to 10% of body weight through calorie restriction and physical activity has significant health benefits. When the risk is high, drug therapy for elevations in blood pressure, low-density lipoprotein cholesterol (LDL-C), and glucose levels should be incorporated into the regimen. The nursing process is applied to the metabolic syndrome by initially focusing on the root causes of improper nutrition and inadequate physical activity, as detailed in Table 25–3. TABLE 25–3 Data from Carpenito-Moyet LJ: Nursing diagnosis: application to clinical practice, ed 10, Philadelphia, 2004, JB Lippincott. Metabolically distinct genetic influences play a pivotal role in geriatric diabetes and require a different approach (Meneilly, 2006). Often starting with metabolic syndrome, the disease ultimately produces dysfunction and failure of various organs, such as the heart, kidneys, nerves, eyes, and blood vessels (Grundy et al, 2005). Age-related changes combine with genetics and lifestyle factors to produce a hyperglycemic state. Current evidence suggests that the hyperglycemia of type 2 diabetes mellitus is caused by impaired carbohydrate metabolism, changes in pulsatile insulin release, and resistance to insulin-mediated glucose disposal (Meneilly, 2007). As with metabolic syndrome, the most important variables associated with type 2 diabetes mellitus are obesity and insulin resistance. Starting with a compensatory hyperinsulinemia that affects insulin receptors on target tissues, which leads to insulin resistance that produces hyperglycemia, type 2 diabetes mellitus is a disorder of relative insulin insufficiency. The pathophysiology of type 2 diabetes mellitus in contrast to type 1 diabetes mellitus involves defects in the cell membrane, receptors, or intracellular pathways (Figs. 25–2 and 25–3). Genetic defects of beta cell function and insulin action interact with lifestyle factors to make diabetes one of the most common chronic conditions: It affects 40% of the elderly population (Parikh & Munshi, 2007). At the time of diagnosis (Box 25–2), uncontrolled type 2 diabetes mellitus may be associated with symptoms of excessive thirst, hunger, and urination (i.e., polydipsia, polyphagia, and polyuria, respectively). However, the older individual with type 2 diabetes often does not have classic symptomatology and will not complain of weight loss or fatigue along with these classic symptoms (Beers & Jones, 2005). Often a newly diagnosed older individual will describe symptoms of fatigue, blurred vision, weight change (gain or loss), and infections. When questioned, both men and women often attribute these changes to “aging.” Individuals are often diagnosed with diabetes during a concurrent infection, such as a major foot or leg wound, vaginitis, or urinary tract infection, or they may be seen with impotence, numbness of the extremities, or changes in vision. Current medical management focuses on the disease process and utilizes multiple medication classes to control hyperglycemia if greater than 5 years of longevity is expected (Munshi & Lipsitz, 2007). Data from recent research indicate sulfonylureas, insulin, and biguanides do not prevent a loss of beta cell function, so current therapy recommendations use drug combinations that include thiazolidinediones to preserve beta cell function while controlling serum glucose levels (Fig. 25–4). Five different oral drug classes are currently available for use in diabetes management (Box 25–3). Some medications prescribed for comorbid problems can make glucose control more difficult. The nursing process in type 2 diabetes mellitus addresses the core defects of impaired insulin secretion and insulin action, as well as prevention of vascular and microvascular complications of the eye, heart, kidneys, and feet (see Fig. 25–3). TLCs are incorporated into the geriatric plan of care based on the client’s cognitive capacity and functional limitations. Assessment of current living conditions is essential. The nurse should ask if the individual lives alone or with others, if living arrangements afford the ability to prepare food, and if there are adequate financial resources for food and shelter. Older adults who live alone may eat little and be malnourished because of social isolation or functional impairments (Munshi & Lipsitz, 2007). The nurse should determine whether transportation to health care services is available to the older adult client. Type 2 diabetes mellitus is associated with increased depression and memory problems in older adults (Brown, Meltzer, Chin, & Huang, 2008). These problems are often aggravated by uncontrolled diabetes or hyperglycemia. It is important for the nurse to evaluate current and past blood glucose results. The nurse should assess both the older adult’s ability to remember simple facts and his or her mood and level of anxiety. For example, the nurse can ask a client to explain content that was just presented. If the client cannot recall, the nurse needs to determine whether there is a learning or memory problem. Memory testing can be accomplished simply by asking clients to repeat number sequences or by making a short- or long-term memory assessment (see Chapter 4). The nurse should ask the older client about neurologic symptoms such as numbness, tingling, blurred vision, headaches, and the inability to sense temperature, especially in the feet. Nursing diagnoses for an older client with type 2 diabetes mellitus include • Altered nutrition: more than body requirements related to overeating habits or lack of regular exercise patterns • Altered nutrition: less than body requirements related to inadequate intake of nutrients or metabolic imbalance • Altered tissue perfusion related to decreased or interrupted arterial flow • Sexual dysfunction related to metabolic alterations • Altered thought processes related to metabolic alteration or feelings of distress • Knowledge deficit: diabetes self-management and skills related to lack of exposure • Risk for impaired skin integrity related to impaired circulation • Risk for ineffective individual coping related to lack of social support or feelings of distress 1. The client follows the plan of care by taking action on the basis of professional advice as evidenced by a. Reports of following the prescribed regimen b. Correct modification of the regimen as directed by a health professional 2. The client shows evidence of successful individual coping as evidenced by 3. The client demonstrates increased knowledge of the American Diabetes Association (ADA) diet, as evidenced by
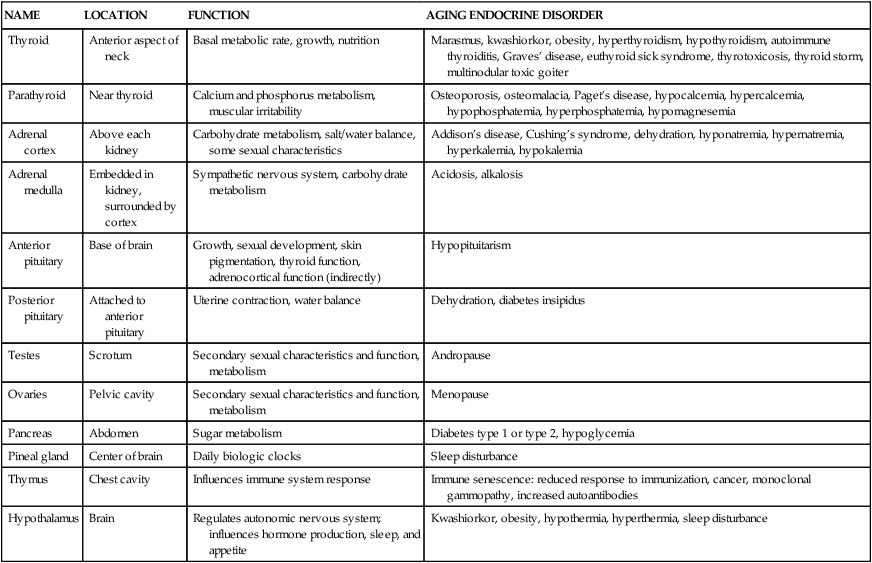
Endocrine Function
Endocrine Physiology in Older Adults
NAME
LOCATION
FUNCTION
AGING ENDOCRINE DISORDER
Thyroid
Anterior aspect of neck
Basal metabolic rate, growth, nutrition
Marasmus, kwashiorkor, obesity, hyperthyroidism, hypothyroidism, autoimmune thyroiditis, Graves’ disease, euthyroid sick syndrome, thyrotoxicosis, thyroid storm, multinodular toxic goiter
Parathyroid
Near thyroid
Calcium and phosphorus metabolism, muscular irritability
Osteoporosis, osteomalacia, Paget’s disease, hypocalcemia, hypercalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia
Adrenal cortex
Above each kidney
Carbohydrate metabolism, salt/water balance, some sexual characteristics
Addison’s disease, Cushing’s syndrome, dehydration, hyponatremia, hypernatremia, hyperkalemia, hypokalemia
Adrenal medulla
Embedded in kidney, surrounded by cortex
Sympathetic nervous system, carbohydrate metabolism
Acidosis, alkalosis
Anterior pituitary
Base of brain
Growth, sexual development, skin pigmentation, thyroid function, adrenocortical function (indirectly)
Hypopituitarism
Posterior pituitary
Attached to anterior pituitary
Uterine contraction, water balance
Dehydration, diabetes insipidus
Testes
Scrotum
Secondary sexual characteristics and function, metabolism
Andropause
Ovaries
Pelvic cavity
Secondary sexual characteristics and function, metabolism
Menopause
Pancreas
Abdomen
Sugar metabolism
Diabetes type 1 or type 2, hypoglycemia
Pineal gland
Center of brain
Daily biologic clocks
Sleep disturbance
Thymus
Chest cavity
Influences immune system response
Immune senescence: reduced response to immunization, cancer, monoclonal gammopathy, increased autoantibodies
Hypothalamus
Brain
Regulates autonomic nervous system; influences hormone production, sleep, and appetite
Kwashiorkor, obesity, hypothermia, hyperthermia, sleep disturbance
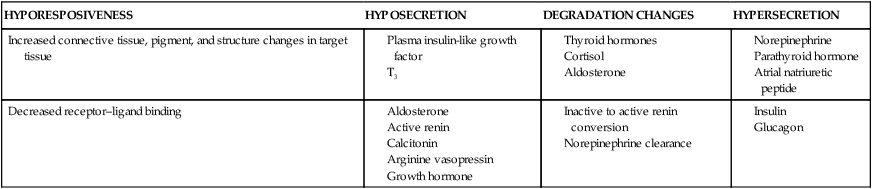
HYPORESPOSIVENESS
HYPOSECRETION
DEGRADATION CHANGES
HYPERSECRETION
Increased connective tissue, pigment, and structure changes in target tissue
Decreased receptor–ligand binding
Andropause and Menopause
Adrenopause
Somatopause
Common Endocrine Pathophysiology in Older Adults
The Metabolic Syndrome: Diabetes Continuum
Pathophysiology
Medical Management
Nursing Process Applied to Metabolic Syndrome
Type 2 Diabetes Mellitus
Pathophysiology
Signs and Symptoms
Medical Management
Nursing Management
![]() Assessment
Assessment
![]() Diagnosis
Diagnosis
![]() Planning and Expected Outcomes
Planning and Expected Outcomes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endocrine Function
Get Clinical Tree app for offline access