Introduction
A small but significant proportion of women develop complications which may threaten their lives or those of their babies. Emergency situations can be very disturbing and provoke a range of emotional responses and consequences for all involved (World Health Organization (WHO), 2003). Acute units must have comprehensive multidisciplinary facilities for rapid response to obstetric emergencies. In birth centres and at home births the midwife is responsible for emergency measures and prompt transfer (Department of Health (DoH), 2004). Regular skills drills have been shown to improve outcomes (Paxton et al., 2005).
Clear, calm explanations of the emergency procedure and the risks involved will help to reduce anxiety for the woman and her partner. Be respectful of the woman’s dignity, be aware that her choices may be reduced, acknowledge her fears and give sensitive responses to her needs (WHO, 2003).
‘Even when serious emergencies occur, midwives can do much to create an environment which respects the woman as a person with feelings and emotions rather than an object to be rushed to theatre.’ (Weston, 2001).
Cord prolapse and cord presentation
Cord presentation means that a loop of umbilical cord lies below the presenting part of the fetus, with intact membranes. If the membranes rupture this is known as a cord prolapse. The prolapse can be occult, i.e. alongside the presenting part, or frank, where the cord escapes through the cervix and may even be visible outside the vagina.
Incidence and facts
- Cord prolapse is reported as 0.4% in vertex presentations, 0.5% in frank breeches, 4–6% in complete breeches and 15–18% in footling breeches (American Academy of Family Physicians (AAFP), 2004).
- 50% of cord prolapse cases occur following artificial rupture of membranes (ARM) (Prabulos & Philipson, 1998).
- Umbilical cord prolapse is associated with poor perinatal outcomes, even when emergency delivery facilities are available (Prabulos & Philipson, 1998).
- Squire (2002) suggests that mortality is predominantly associated with congenital abnormities and prematurity rather than birth asphyxia per se.
- Occult cord prolapse is associated with less perinatal morbidity than frank prolapse (Prabulos & Philipson, 1998).
- In only 41% of cases, electronic fetal monitoring aided the diagnosis of cord prolapse (Murphy & MacKenzie, 1995).
Associated risk factors
- Any situation where the presenting part may not engage well in the pelvis, e.g. high presenting part, high parity, multiple birth, polyhydramnios, small/preterm baby, malpresentation (e.g. breech) (McGeown, 2001), ARM with a high presenting part (AAFP, 2004).
- Long umbilical cord (AAFP, 2004).
Signs and symptoms of cord prolapse
- Visible cord may protrude from vagina.
- Cord felt (often pulsating) on vaginal examination.
- Fetal bradycardia/prolonged late decelerations following rupture of membranes, possibly with fresh meconium liquor.
Practice recommendations/manoeuvres
- Call for help. If at home/birthing centre, call paramedic ambulance even if delivery appears imminent; if in second stage, also call midwife ventouse/forceps practitioner (if available). Give accurate and concise information when communicating with the receiving obstetric unit.
- Maintain pressure on presenting part. Keep the fingers in the vagina firmly pushing on the presenting part, particularly during contractions, to relieve cord compression until urgent delivery is achieved (AAFP, 2004). If achievable maintain pressure during ambulance transfer or while the woman is wheeled to a birth room/theatre (AAFP, 2004). This can be uncomfortable and stressful for whoever is performing the pressure.
- Remain calm and briefly explain to the woman and her partner what is happening and what is required of them. The clinician conducting internal pressure cannot participate in other activities and is ideally situated to offer supportive reassurance and explanations to the woman and her partner during this frightening situation.
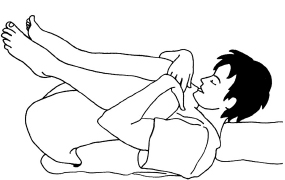
Fig. 16.1 Knee-chest position.
- Position the mother. The all fours/knee-chest position reduces pressure caused by the presenting part (see Fig. 16.1). It is possibly the most effective position, but can be uncomfortable and undignified for the woman. Cover her lower half for modesty.
- Alternative positions:
 Trendelenburg. The woman lies on her back with a 30° tilt using a wedge to prevent aortocaval compression with a head down tilt, if possible, to relieve pressure.
Trendelenburg. The woman lies on her back with a 30° tilt using a wedge to prevent aortocaval compression with a head down tilt, if possible, to relieve pressure. Exaggerated Sims. The woman lies on her left side with her upper leg flexed and the knee resting on the bed.
Exaggerated Sims. The woman lies on her left side with her upper leg flexed and the knee resting on the bed.- Gently replace the cord into the vagina if it is protruding, but do not attempt to replace into the uterus (AAFP, 2004). Keep the cord warm and wet using sterile gauze or a cloth and polythene bag if replacement is impossible (Davis, 1997) as cold air or excessive handling can cause spasm and resultant reduced oxygen delivery.
- Fill the bladder. Some success is reported from instilling 500–700 ml of saline into the bladder (AAFP, 2004). This may relieve cord compression and inhibit uterine activity, in cases where theatre is not immediately available. Prolonged overdistension of the bladder should be avoided.
- Deliver urgently. In the first stage of labour, caesarean section (CS) is normally performed. However if in advanced second stage, encourage the woman to push. Most primigravidae are unlikely to be able to deliver quickly, and an episiotomy and instrumental delivery are probable. Multigravid women may also require such intervention, but quick spontaneous birth may be achieved if they push hard: consider an upright position, as the risk of cord compression may be offset by the improved pushing. Close fetal heart monitoring is vital.
- Assistance with resuscitation. A baby with a frank cord prolapse is likely to require intensive resuscitation; therefore, paediatric support should be summoned. Appropriate help should be summoned at a birthing centre or at home.
Amniotic fluid embolism
Amniotic fluid embolism (AFE) is a rare but catastrophic condition, which is usually fatal (AAFP, 2004). A woman may collapse rapidly with no clear diagnosis at the time. AFE is an anaphylactic type reaction to amniotic fluid that has entered the woman’s circulation (AAFP, 2004). This results in left ventricular failure and pulmonary vasospasm resulting in acute lung injury. Clotting factors are also activated with disseminated intravascular coagulation (DIC) commonly resulting (Davis, 1999; Fahy, 2001; AAFP, 2004). The rapidly deteriorating situation can be extremely stressful for staff and birth partners, particularly as death so often results.
Incidence and facts
- Incidence of AFE is reported as 1:20 000 deliveries (AAFP, 2004), with a 26.4–61% mortality rate (AAFP, 2004; Confidential Enquiry into Maternal and Child Health (CEMACH), 2004). The perinatal mortality rate is 40%.
- CEMACH (2004) reports 5 deaths from AFE, with 19 cases reported to the National AFE Register.
- Risk factors include multiparity, abruption, intrauterine fetal demise, tumultuous labour and oxytocic hyperstimulation (AAFP, 2004). AFE remains unpredictable, rapidly progressive and unpreventable (CEMACH, 2004).
- Diagnosis may be confirmed by the presence of fetal squames and lanugo hair in the pulmonary vasculature at post-mortem (CEMACH, 2004), or reliant on clinical observations if autopsy is unavailable (AAFP, 2004). Fahy (2001) suggests that the more knowledgeable clinicians become about AFE, the more frequently it is diagnosed.
Signs and symptoms of AFE
(See Table 16.1.)
Table 16.1 Signs and symptoms of AFE (Fahy, 2001).
| Sign/symptom | % of women |
| Hypotension (shock) | 100 |
| Fetal distress (if undelivered) | 100 |
| Pulmonary oedema or adult respiratory distress syndrome | 93 |
| Cardiopulmonary arrest | 86 |
| Cyanosis | 83 |
| Coagulopathy | 83 |
| Dyspnoea (difficult or laboured breathing) | 49 |
| Seizure | 48 |
- AFE manifests itself with the woman gasping for breath, developing rapid hypotension and shock symptoms, and usually going into cardiac arrest (AAFP, 2004). A behavioural change may precede collapse, as hypoxia and toxic confusional state develops (CEMACH, 2004).
- Sometimes haemorrhage due to DIC is the initial presentation (Davis, 1999).
Practice recommendations
- Call for help/transfer to hospital.
- Think ABC. Airway, Breathing and Circulation (see Box 16.4 and Chapter 17).
- Early input of co-ordinated care from the resuscitation team and consultant anaesthetist, obstetrician and haematologist (CEMACH, 2004). Rapid intensive therapy unit transfer is recommended and may increase survival (CEMACH, 2004).
- Deliver baby by CS as rapidly as possible in the event of maternal cardiac arrest (Managing Obstetric Emergencies and Trauma (MOET), 2003). Obstetricians do not need to prepare a sterile field and should go for the quickest incision possible (Bobrowski, 1994).
- Aftercare. The woman will require intensive care with supportive treatment (AAFP, 2004).
Uterine rupture
Uterine dehiscence is defined as disruption of the uterine muscle with intact serosa (Royal College of Paediatrics and Child Health (RCOG), 2007). The more serious condition of uterine rupture is defined as disruption of the uterine muscle extending to and involving the uterine serosa or disruption of the uterine muscle with extension to the bladder or broad ligament (RGOG, 2007). Occasionally these events occur antenatally, but more often during labour, birth or prior to delivery of the placenta (RCOG, 2007).
For additional information see Box 16.1.
Incidence and facts
- Uterine rupture is rare. Incidence in women with an unscarred uterus is reported as 2 per 10 000 and with a scarred uterus 0.5 to 2 per 10 000 deliveries (RCOG, 2007).
- 50% of cases occur in women with no uterine scar, mainly multiparous women (Enkin, 2000).
- 13% uterine ruptures occur outside the hospital setting (AAFP, 2004).
- There is no single clinical feature indicative of uterine rupture: diagnosis is ultimately confirmed at CS or postpartum laparotomy (RCOG, 2007).
- Early recognition and diagnosis may improve outcomes (AAFP, 2004).
Associated risk factors
- 50% cases associated with previous uterine surgery including CS (AAFP, 2004).
- Other associated causes: inappropriate use of prostaglandins/oxytocin to induce/ augment labour (National Institute for Health and Clinical Excellence (NICE), 2004, AAFP, 2004), trauma caused by high-cavity forceps, manual manipulation for unstable lie, manual removal of placenta, road traffic incident or other blunt trauma including physical assault (Kroll & Lyne, 2002).
Signs and symptoms of uterine rupture are given in Box 16.1.
Practice recommendations
- Midwifery care: call for help/transfer to hospital.
- Discontinue intravenous (IV) oxytocin if in progress (AAFP, 2004).
- Administer oxygen and IV fluids rapidly.
Box 16.1 Signs and symptoms of uterine rupture.
- Sudden uterine or scar pain (AAFP, 2004).
- Chest or shoulder tip pain (RCOG, 2007).
- A feeling of ‘giving way’ (Silverton, 1993).
- Lower abdominal pain may come with a contraction, or be constant and unrelenting (AAFP, 2004).
- The woman may find it too painful to have her uterus touched or palpated.
- Pain may decrease after the rupture (WHO, 2003).
- Solid, tonic uterus or abnormal uterine shape (WHO, 2003).
- Contractions may stop or dwindle (AAFP, 2004).
- Abnormal cardiotocograph may occur (RCOG, 2007) culminating in prolonged fetal bradycardia (AAFP, 2004).
- Recession of the presenting part (RCOG, 2007) or suprapubic bulging (AAFP, 2004).
- Easily palpable fetal parts (WHO, 2003).
- Tachycardia (AAFP, 2004).
- Hypotension (RCOG, 2007).
- Sudden onset of shortness of breath (RCOG, 2007).
- Look cold and clammy.
- Appear restless, agitated or withdrawn.
- Say she is frightened and that something is wrong.
- Vomit.
- Fresh vaginal bleeding or blood-stained amniotic fluid may be seen.
- Haematuria may develop (RCOG, 2007).
- Following delivery a ruptured uterus may rise as it fills with blood.
- Deliver the baby by instrumental means or proceed to immediate CS (AAFP, 2004).
- Repair the uterus immediately in theatre. Haemorrhage is likely. If bleeding is uncontrollable, hysterectomy may be necessary (Bakshi & Meyer, 2000).
Aftercare
- Closely monitor the woman following surgery as she is at risk of postpartum haemorrhage (RCOG, 2007). An IV oxytocin infusion is advisable post-delivery. In severe cases the mother and baby may require intensive care. Perinatal morbidity is more common in cases of complete displacement of the baby into the abdominal cavity due to uterine rupture (AAFP, 2004).
Shoulder dystocia
Shoulder dystocia is one of the most serious birth emergencies. It is caused by the impaction of the anterior shoulder of the fetus against the maternal symphysis pubis, or less commonly the posterior fetal shoulder on the sacral promontory, after delivery of the head (RCOG, 2005), requiring additional obstetric manoeuvres to release the shoulders (RCOG, 2005). Unless the baby is born within a few minutes of its head emerging, it is likely to die. Shoulder dystocia cannot be predicted (WHO, 2003); all midwives must be able to recognise and manage this emergency promptly (Brown, 2002).
Incidence and facts
- Shoulder dystocia occurs in 0.3–1% of babies weighing 2.5–4 kg and 5–7% of babies weighing 4–4.5 kg (AAFP, 2004).
- Over 50% of shoulder dystocias occur in normal sized babies with no identifiable risk factors (AAFP, 2004).
- Preconception and prenatal risk factors have extremely poor predictive value and therefore in clinical practice do not facilitate accurate, reliable prediction of shoulder dystocia (Gherman, 2002).
- Morbidity for the baby includes obstetric brachial plexus palsy (OBPP) injuries in 7–20% following dystocia, with 1–2% of those sustaining permanent injury (AAFP, 2004). Hypoxia, fractures of the clavicle/humerus, bruising and soft tissue damage may occur, and in severe cases, fetal death may result.
- Litigation may result from OBPP: 40–60 births from 1993 to 1997 resulted in claims (NHSLA, 2003).
- Morbidity for the woman includes trauma, blood loss, bruising to the perineum/ genital tract and surrounding tissues, episiotomy/serious tears, psychological trauma, as well as, in severe cases, grief at the death of her baby.
- Simulated training sessions have been found to improve performance in shoulder dystocia management (Deering et al., 2004).
Associated risk factors
All shoulder dystocia-associated risk factors have poor predictive value in clinical practice (WHO, 2003).
Antenatal associated risks:
- Macrosomia (AAFP, 2004) and previous baby >4.0 kg (CEMACH, 2004).
- Maternal diabetes, short stature and abnormal pelvic anatomy (AAFP, 2004).
- Previous instrumental delivery (AAFP, 2004).
- Post-dates pregnancy (AAFP, 2004).
- Previous shoulder dystocia (AAFP, 2004).
Intrapartum-associated risks:
- Prolonged first or second stage of labour (RCOG, 2005).
- Birthing semi-recumbent on a bed can restrict the movement of the coccyx and sacrum contributing to ‘bed-birth dystocia’ (Mortimore & McNabb, 1998; McGeown, 2001).
- Oxytocin augmentation (RCOG, 2005).
- Instrumental delivery (AAFP, 2004).
Recognising shoulder dystocia
Shoulder dystocia is usually preceded by a slow ‘bobbing’ delivery of the baby’s head; the baby’s chin then retracts against the perineum and ‘turtlenecks’ (AAFP, 2004). With the next contraction the baby will not deliver as its anterior shoulder is impacted against the symphysis pubis bone, due to the shoulder (bisacromial) diameter exceeding the diameter of the pelvic inlet (AAFP, 2004).
Beware of overdiagnosing shoulder dystocia. Sometimes anxious clinicians start to worry after only 1 or 2 minutes. Think: ‘has there been a contraction yet?’ Two minutes can seem like a long time, but no baby will be compromised at this point: this is quite normal. Spontaneous restitution of the shoulders may take one or two contractions. Premature traction without a contraction may give the false impression that dystocia is occurring.
Practice recommendations
Upright birthing positions improve the alignment of the mobile pelvic bones and improve the shape and capacity of the pelvis, optimising the chances of a ‘good fit’ between baby and pelvis (Simpkin & Ancheta, 2005). Common sense suggests that any woman at risk of shoulder dystocia should be discouraged from birthing in the semi-recumbent position.
Changing the woman’s position in itself can be beneficial in preventing/shifting impacted shoulders.
Do not clamp and cut a nuchal cord. This will cut off the only oxygen supply the baby has, and hypoxia will rapidly follow.
Avoid excessive traction. Once shoulder dystocia is diagnosed, do not put any further traction on the head. A previously healthy fetus will withstand up to 10 minutes of complete anoxia before there is a significant risk of brain damage (Pasternak, 1993). Whilst a fetus who has experienced prior fetal distress may not endure more than 5 minutes of anoxia (Confidential Enquiry into Stillbirths and Deaths in Infancy (CESDI), 1998) and it may be justifiable to resume traction if other manoeuvres have repeatedly failed, any OBPP injury following a head-to-body delivery of, say, 2–3 minutes will probably be judged in court to have been due to excessive traction (Johnson, 2005). Performing the manoeuvres correctly will certainly take more than 2–3 minutes.
Avoid fundal pressure which could increase shoulder impaction and cause serious injuries, including uterine rupture, haemorrhage and even maternal death.
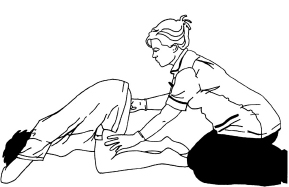
Use of systematic manoeuvres. Most shoulder dystocias will resolve with these manoeuvres. Prophylactic use of McRoberts or other manoeuvres prior to clinical diagnosis of shoulder dystocia requires further evaluation (Poggi, 2004). Many midwives might respond however that prevention happens all the time: optimal positioning in the second stage, e.g. squatting/all fours (which might be described as physiological
Fig. 16.2 McRoberts manoeuvre (side view).