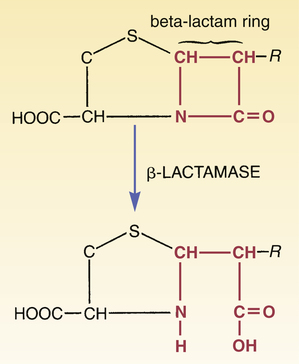
CHAPTER 84 Because they have a beta-lactam ring in their structure (Fig. 84–1), the penicillins are known as beta-lactam antibiotics. The beta-lactam family also includes the cephalosporins, carbapenems, and aztreonam (see Chapter 85). All of the beta-lactam antibiotics share the same mechanism of action: disruption of the bacterial cell wall. Penicillins weaken the cell wall by two actions: (1) inhibition of transpeptidases and (2) disinhibition (activation) of autolysins. Transpeptidases are enzymes critical to cell wall synthesis. Specifically, they catalyze the formation of cross-bridges between the peptidoglycan polymer strands that form the cell wall, and thereby give the cell wall its strength (Fig. 84–2). Autolysins are bacterial enzymes that cleave bonds in the cell wall. Bacteria employ these enzymes to break down segments of the cell wall to permit growth and division. By simultaneously inhibiting transpeptidases and activating autolysins, the penicillins (1) disrupt synthesis of the cell wall and (2) promote its active destruction. These combined actions result in cell lysis and death. The molecular targets of the penicillins (transpeptidases, autolysins, other bacterial enzymes) are known collectively as penicillin-binding proteins (PBPs). These molecules are so named because penicillins must bind to them to produce antibacterial effects. As indicated in Figure 84–3, PBPs are located on the outer surface of the cytoplasmic membrane. More than eight different PBPs have been identified. Of these, PBP1 and PBP3 are most critical to penicillin’s antibacterial effects. Bacteria express PBPs only during growth and division. Accordingly, since PBPs must be present for penicillins to work, these drugs work only when bacteria are growing. As indicated in Figure 84–3, the cell envelope of gram-positive bacteria has only two layers: the cytoplasmic membrane plus a relatively thick cell wall. Despite its thickness, the cell wall can be readily penetrated by penicillins, giving them easy access to PBPs on the cytoplasmic membrane. As a result, penicillins are generally very active against gram-positive organisms. The gram-negative cell envelope has three layers: the cytoplasmic membrane, a relatively thin cell wall, and an additional outer membrane (see Fig. 84–3). Like the gram-positive cell wall, the gram-negative cell wall can be easily penetrated by penicillins. The outer membrane, however, is difficult to penetrate. As a result, only certain penicillins (eg, ampicillin) are able to cross it and thereby reach PBPs on the cytoplasmic membrane. Beta-lactamases are enzymes that cleave the beta-lactam ring, and thereby render penicillins and other beta-lactam antibiotics inactive (Fig. 84–4). Bacteria produce a large variety of beta-lactamases; some are specific for penicillins, some are specific for other beta-lactam antibiotics (eg, cephalosporins), and some act on several kinds of beta-lactam antibiotics. Beta-lactamases that act selectively on penicillins are known as penicillinases. Penicillinases are synthesized by gram-positive and gram-negative bacteria. Gram-positive organisms produce large amounts of these enzymes, and then export them into the surrounding medium. In contrast, gram-negative bacteria produce penicillinases in relatively small amounts, and, rather than exporting them to the environment, secrete them into the periplasmic space (see Fig. 84–3). Certain bacterial strains, known collectively as methicillin-resistant Staphylococcus aureus (MRSA), have a unique mechanism of resistance: production of PBPs with a low affinity for penicillins and all other beta-lactam antibiotics. How did MRSA develop this ability? By acquiring genes that code for low-affinity PBPs from other bacteria. Infection with MRSA and its management are discussed in Box 84–1. All of the penicillins are derived from a common nucleus: 6-aminopenicillanic acid. As shown in Figure 84–1, this nucleus contains a beta-lactam ring joined to a second ring. The beta-lactam ring is essential for antibacterial actions. Properties of individual penicillins are determined by additions made to the basic nucleus, primarily at the site labeled R. These modifications determine (1) affinity for PBPs, (2) resistance to penicillinases, (3) ability to penetrate the gram-negative cell envelope, (4) resistance to stomach acid, and (5) pharmacokinetic properties. The most useful classification of penicillins is based on antimicrobial spectrum. When classified this way, the penicillins fall into four major groups: (1) narrow-spectrum penicillins that are penicillinase sensitive, (2) narrow-spectrum penicillins that are penicillinase resistant (antistaphylococcal penicillins), (3) broad-spectrum penicillins (aminopenicillins), and (4) extended-spectrum penicillins (antipseudomonal penicillins). Table 84–1 lists the members of each group and their principal target organisms. TABLE 84–1 Classification of the Penicillins *Methicillin is no longer used in the United States. †Ticarcillin, by itself, is no longer available in the United States, but ticarcillin combined with clavulanic acid is still available. Penicillin G (benzylpenicillin) was the first penicillin available and will serve as our prototype for the penicillin family. This drug is often referred to simply as penicillin. Penicillin G is bactericidal to a number of gram-positive bacteria as well as to some gram-negative bacteria. Despite the introduction of newer antibiotics, penicillin G remains a drug of choice for many infections. Its structure is shown in Figure 84–1.
Drugs that weaken the bacterial cell wall I: penicillins
Introduction to the penicillins
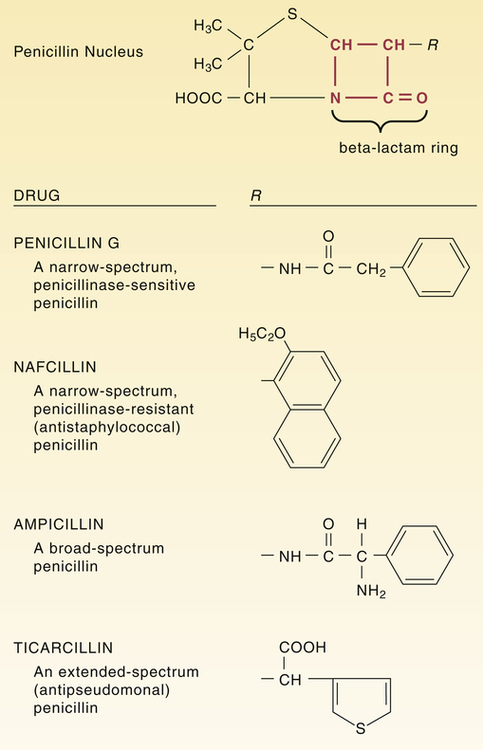
 Structural formulas of representative penicillins.
Structural formulas of representative penicillins.
The unique structure of individual penicillins is determined by the side chain coupled to the penicillin nucleus at the position labeled R. This side chain influences acid stability, pharmacokinetic properties, penicillinase resistance, and ability to bind specific penicillin-binding proteins.
Mechanism of action
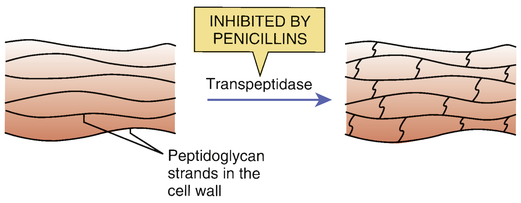
 Inhibition of transpeptidase by penicillins.
Inhibition of transpeptidase by penicillins.
The bacterial cell wall is composed of long strands of a peptidoglycan polymer. As depicted, transpeptidase enzymes create cross-bridges between the peptidoglycan strands, giving the cell wall added strength. By inhibiting transpeptidases, penicillins prevent cross-bridge synthesis and thereby weaken the cell wall.
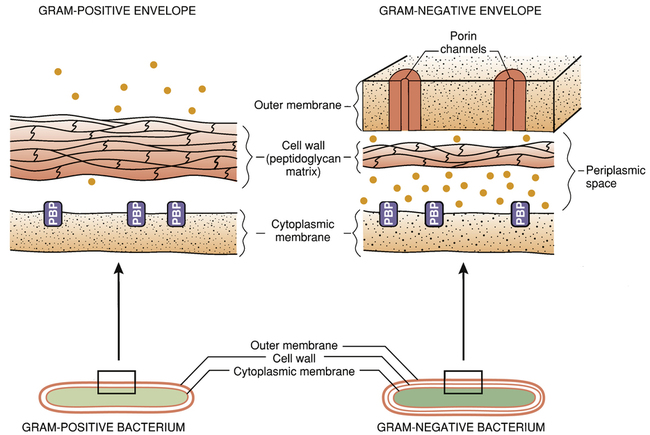
 The bacterial cell envelope.
The bacterial cell envelope.
Note that the gram-negative cell envelope has an outer membrane, whereas the gram-positive envelope does not. The outer membrane of the gram-negative cell envelope prevents certain penicillins from reaching their target molecules. (PBP = penicillin-binding protein [transpeptidases and other penicillin target molecules], • = beta-lactamases.)
Mechanisms of bacterial resistance
The gram-negative cell envelope
Penicillinases (beta-lactamases)
Altered penicillin-binding proteins
Chemistry
Classification

Penicillin Class
Drug
Clinically Useful Antimicrobial Spectrum
Narrow-spectrum penicillins: penicillinase sensitive
Penicillin G
Penicillin V
Streptococcus species, Neisseria species, many anaerobes, spirochetes, others
Narrow-spectrum penicillins: penicillinase resistant (antistaphylococcal penicillins)
Methicillin*
Nafcillin
Oxacillin
Dicloxacillin
Staphylococcus aureus
Broad-spectrum penicillins (aminopenicillins)
Ampicillin
Amoxicillin
Haemophilus influenzae, Escherichia coli, Proteus mirabilis, enterococci, Neisseria gonorrhoeae
Extended-spectrum penicillins (antipseudomonal penicillins)
Ticarcillin†
Piperacillin
Same as broad-spectrum penicillins plus Pseudomonas aeruginosa, Enterobacter species, Proteus (indole positive), Bacteroides fragilis, many Klebsiella
Properties of individual penicillins
Penicillin G

Drugs that weaken the bacterial cell wall I: penicillins
Get Clinical Tree app for offline access