CHAPTER 64 Drugs that alter uterine function fall into two major groups: oxytocic drugs and tocolytic drugs. The oxytocic drugs, also known as uterotonic drugs, stimulate uterine contraction. In contrast, the tocolytic drugs cause uterine relaxation. Clinical applications of the oxytocic and tocolytic drugs are summarized in Table 64–1. TABLE 64–1 Applications of Selected Tocolytic and Oxytocic Drugs *Not available in the United States. All of the drugs employed to suppress preterm labor are tocolytics. That is, they all promote uterine relaxation, and thereby delay delivery. Be aware, however, that benefits are limited: These drugs can only suppress labor briefly, not long term. On average, delivery is postponed by only 48 hours. Hence, birth still takes place prior to term. If tocolytics don’t permit pregnancy to reach term, what are they good for? The answer: Almost nothing—if they are used alone. However, when tocolytics are combined with glucocorticoids, which accelerate fetal lung development (see Chapter 107), the outcome can be improved: Infants experience less respiratory distress syndrome, intraventricular hemorrhage, and mortality. Tocolytics also buy time to treat infection, if present. Unfortunately, tocolytic drugs can pose a risk to the fetus. Accordingly, the ultimate goal of treatment is to extend fetal time in the womb, but without causing significant fetal or neonatal harm. Contraction of the myometrium (uterine smooth muscle) is regulated by multiple mediators, including beta-adrenergic agonists, oxytocin, and prostaglandins (Fig. 64–1). As a result, there are multiple ways in which drugs can suppress uterine activity. However, although these drugs work through different mechanisms, they all have one thing in common: Ultimately, they all decrease the availability of phosphorylated light-chain (LC) myosin, the form of myosin that interacts with actin to cause contraction. As indicated in Figure 64–1, four classes of tocolytic drugs—beta-adrenergic agonists, calcium channel blockers, cyclooxygenase (COX) inhibitors, and oxytocin receptor antagonists—work to reduce the activity of myosin LC kinase, the enzyme that converts myosin to its phosphorylated form. A fifth group—the nitric oxide donors—work to increase the activity of myosin LC phosphatase, the enzyme that removes phosphate from myosin, thereby converting it to its inactive form. Note also that three drug groups—COX inhibitors, oxytocin receptor antagonists, and calcium channel blockers—decrease the release of calcium from the sarcoplasmic reticulum (SR). As indicated in the figure, calcium combines with calmodulin to form a complex that increases myosin light-chain kinase activity. Hence, in the absence of sufficient free calcium, myosin LC kinase activity declines, causing the phosphorylation of myosin to decline as well. Multiple drugs can suppress preterm labor. Options include terbutaline (a beta2-adrenergic agonist), nifedipine (a calcium channel blocker), and COX inhibitors (eg, indomethacin). All of these drugs appear equally good at suppressing labor, and hence there is no obvious “first-choice” agent among them. Accordingly, selection is based primarily on side effects, which are summarized in Table 64–2. Interestingly, none of the drugs currently employed to suppress preterm labor has been approved for this use by the Food and Drug Administration (FDA). TABLE 64–2 Adverse Effects of Tocolytic Drugs Terbutaline, used primarily for asthma (see Chapter 76), is a selective beta2 agonist that can effectively suppress preterm labor. By activating beta2 receptors in the uterus, terbutaline increases production of cyclic AMP (cAMP), a mediator that leads to suppression of myosin light-chain kinase activity. The result is a decrease in both the intensity and frequency of contractions. Unfortunately, although terbutaline is effective, it poses a significant risk to the mother. Adverse effects result from activating beta1 receptors as well as beta2 receptors. (Although terbutaline is classified as beta2 selective, it can activate beta1 receptors too, albeit less readily than beta2 receptors.) Effects of greatest concern are pulmonary edema, hypotension, and hyperglycemia in the mother, and tachycardia in both the mother and fetus. For suppression of labor, terbutaline is administered subQ, not PO. The initial dosage is 250 mcg every 20 minutes for up to 3 hours. Dosing should stop after 48 hours, and should be interrupted if the maternal heart rate exceeds 120 beats per minute. Although terbutaline can be used to suppress preterm labor, it should not be given to prevent preterm labor. Nifedipine [Adalat, Procardia, others] can suppress preterm labor for at least 48 hours. Efficacy equals that of terbutaline—and safety is superior. How does nifedipine work? It blocks calcium channels, and thereby inhibits entry of calcium into myometrial cells. As a result, release of calcium from the SR is reduced, and hence the activity of myosin light-chain kinase is reduced as well. Maternal side effects, which are rare, include transient tachycardia, facial flushing, headache, dizziness, and nausea. Hypotension may occur in hypovolemic patients. There is some concern that nifedipine may compromise uteroplacental blood flow. In animal studies, calcium channel blockers have caused acidosis, hypoxemia, and hypercapnia in the newborn. To suppress preterm labor, an initial loading dose (30 mg sublingual) is followed by maintenance doses (10 or 20 mg PO) every 4 to 6 hours. The basic pharmacology of calcium channel blockers is discussed in Chapter 45. • Are there any long-term risks to the child? (Experience with diethylstilbestrol, an estrogen that can cause vaginal cancer in women exposed to it in utero, is a warning for caution.) • Do these results apply to women at risk of preterm delivery for reasons other than having a history of the problem? • Why did hydroxyprogesterone only work in some women? (Preterm delivery still occurred in nearly half of the women who were treated.)
Drugs that affect uterine function

Applications
Drug
Trade Name
Delay of Preterm Labor
Induction of Cervical Ripening
Induction of Labor
Control of Postpartum Hemorrhage
Induction of Abortion
Tocolytic Drugs
Beta2-adrenergic Agonist
Terbutaline
Brethine
Calcium Channel Blocker
Nifedipine
Adalat, Procardia, others
Cyclooxygenase Inhibitor
Indomethacin
Indocin
Nitric Oxide Donor
Nitroglycerin
Nitro-Dur, others
Oxytocin Antagonist
Atosiban*
Antocin*
Oxytocic (Uterotonic) Drugs
Prostaglandins
Dinoprostone
Cervidil, Prepidil
 †
†
Misoprostol
Cytotec
 †
†
Carboprost
Hemabate
Oxytocin Receptor Agonist
Oxytocin
Pitocin
Ergot Alkaloids
Ergonovine
Ergotrate
Methylergonovine
Methergine
Drugs for preterm labor
Drugs used to suppress preterm labor
Control of myometrial contraction and mechanisms of tocolytic drug action
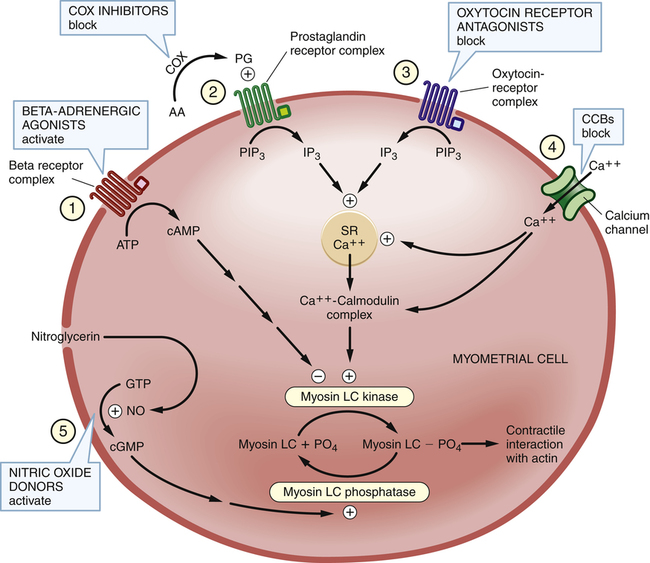
 Control of myometrial contraction and the actions of tocolytic drugs.
Control of myometrial contraction and the actions of tocolytic drugs.
The figure shows five pathways that regulate availability of myosin LC phosphate (myosin LC PO4), the form of myosin needed for contractile interaction with actin. Note that two enzymes—myosin LC kinase and myosin LC phosphatase—play central roles. Four classes of tocolytic drugs (numbers 1, 2, 3, and 4 in the figure) work to reduce the activity of myosin LC kinase, and thereby reduce production of myosin LC phosphate. A fifth class—the nitric oxide donors—increases the activity of myosin LC phosphatase, and thereby stimulates conversion of myosin LC phosphate to its inactive (dephosphorylated) form. Note also the important role played by calcium in controlling the activity of myosin LC kinase. (AA = arachidonic acid, ATP = adenosine triphosphate, cAMP = cyclic adenosine monophosphate, CCBs = calcium channel blockers, cGMP = cyclic guanosine monophosphate, COX = cyclooxygenase, GTP = guanosine triphosphate, IP3 = inositol triphosphate, LC = light-chain, NO = nitric oxide, PG = prostaglandin, PIP3 = phosphatidylinositol triphosphate, PO4 = phosphate, SR = sarcoplasmic reticulum.)
Specific tocolytic drugs

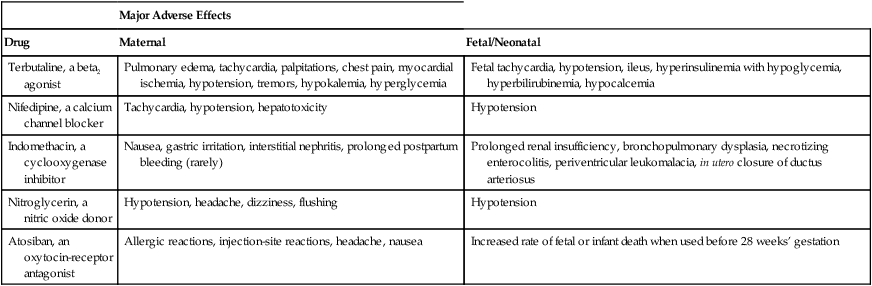
Major Adverse Effects
Drug
Maternal
Fetal/Neonatal
Terbutaline, a beta2 agonist
Pulmonary edema, tachycardia, palpitations, chest pain, myocardial ischemia, hypotension, tremors, hypokalemia, hyperglycemia
Fetal tachycardia, hypotension, ileus, hyperinsulinemia with hypoglycemia, hyperbilirubinemia, hypocalcemia
Nifedipine, a calcium channel blocker
Tachycardia, hypotension, hepatotoxicity
Hypotension
Indomethacin, a cyclooxygenase inhibitor
Nausea, gastric irritation, interstitial nephritis, prolonged postpartum bleeding (rarely)
Prolonged renal insufficiency, bronchopulmonary dysplasia, necrotizing enterocolitis, periventricular leukomalacia, in utero closure of ductus arteriosus
Nitroglycerin, a nitric oxide donor
Hypotension, headache, dizziness, flushing
Hypotension
Atosiban, an oxytocin-receptor antagonist
Allergic reactions, injection-site reactions, headache, nausea
Increased rate of fetal or infant death when used before 28 weeks’ gestation
Terbutaline, a beta2-adrenergic agonist
Nifedipine, a calcium channel blocker
Drugs used to prevent preterm labor
Hydroxyprogesterone caproate
Therapeutic use.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


