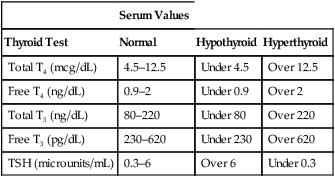
CHAPTER 58 The thyroid gland produces two active hormones: triiodothyronine (T3) and thyroxine (T4, tetraiodothyronine). As shown in Figure 58–1, these hormones have nearly identical structures. The only difference is that T4 contains four atoms of iodine, whereas T3 contains three. The biologic effects of T3 and T4 are qualitatively similar. However, when compared on a molar basis, T3 is much more potent. Synthesis of thyroid hormones takes place in four steps (Fig. 58–2). The circled numbers in the figure correspond with the steps below. • Step 1. Formation of thyroid hormone begins with the active transport of iodide into the thyroid. Under normal conditions, this process produces concentrations of iodide within the thyroid that are 20 to 50 times greater than the concentration of iodide in plasma. When plasma iodide levels are extremely low, intrathyroid iodide content may reach levels that are more than 100 times greater than those in plasma. • Step 2. Following uptake, iodide undergoes oxidation to iodine, the active form of iodide. Iodide oxidation is catalyzed by an enzyme called peroxidase. • Step 3. In this step, activated iodine becomes incorporated into tyrosine residues that are bound to thyroglobulin, a large glycoprotein. As indicated in Figure 58–2, one tyrosine molecule may receive either one or two iodine atoms, resulting in the production of monoiodotyrosine (MIT) or diiodotyrosine (DIT), respectively. • Step 4. In this final step, iodinated tyrosine molecules are coupled. Coupling of one DIT with one MIT forms T3 (step 4A); coupling of one DIT with another DIT forms T4 (step 4B). The functional relationship between the hypothalamus, anterior pituitary, and thyroid is depicted in Figure 58–3. As indicated, thyrotropin-releasing hormone (TRH), secreted by the hypothalamus, acts on the pituitary to cause secretion of thyrotropin (thyroid-stimulating hormone [TSH]). TSH then acts on the thyroid to stimulate all aspects of thyroid function: thyroid size is enlarged, iodine uptake is augmented, and synthesis and release of thyroid hormones are increased. In response to rising plasma levels of T3 and T4, further release of TSH is suppressed. The stimulatory effect of TSH on the thyroid, followed by the inhibitory effect of thyroid hormones on the pituitary, constitutes a negative feedback loop. Several laboratory tests can be used to evaluate thyroid function. Three are described below. Values indicating euthyroid (normal), hypothyroid, and hyperthyroid states are summarized in Table 58–1. TABLE 58–1 Serum Values for Thyroid Function Tests Maternal hypothyroidism can result in permanent neuropsychologic deficits in the child. We have long known that congenital hypothyroidism can cause mental retardation and other developmental problems (see below under Hypothyroidism in Infants). However, it was not until 1999 that researchers demonstrated that maternal hypothyroidism—in the absence of fetal hypothyroidism—can decrease IQ and other aspects of neuropsychologic function in the child. The impact of maternal hypothyroidism is limited largely to the first trimester, a time during which the fetus is unable to produce thyroid hormones of its own. By the second trimester, the fetal thyroid gland is fully functional, and hence the fetus can supply its own hormones from then on. Therefore, to help ensure healthy fetal development, maternal hypothyroidism must be diagnosed and treated very early. Unfortunately, symptoms of hypothyroidism are often nonspecific (irritability, tiredness, poor concentration, etc.) or there may be no symptoms at all. Accordingly, some authorities now recommend routine screening for hypothyroidism as soon as pregnancy is confirmed. If hypothyroidism is diagnosed, replacement therapy should begin immediately. Thyroid hormones are available as pure, synthetic compounds and as extracts of animal thyroid glands. All preparations have qualitatively similar effects. The synthetic preparations are more stable and better standardized than the animal gland extracts. As a result, the synthetics are preferred to the natural products. Properties of thyroid hormone preparations are summarized in Table 58–2. TABLE 58–2
Drugs for thyroid disorders
Thyroid physiology
Chemistry and nomenclature
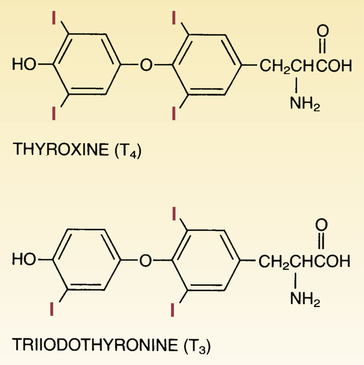
 Structural formulas of the thyroid hormones.
Structural formulas of the thyroid hormones.
Synthesis and fate of thyroid hormones
Synthesis.
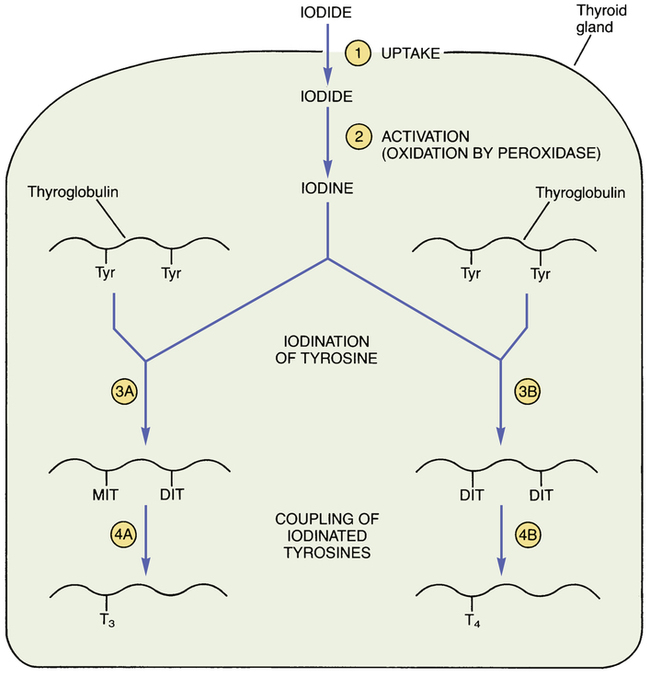
 Steps in thyroid hormone synthesis.
Steps in thyroid hormone synthesis.
The reactions at each step (circled numbers) are explained in the text. (DIT = diiodotyrosine, MIT = monoiodotyrosine, T3 = triiodothyronine, T4 = thyroxine, Tyr = tyrosine.)
Regulation of thyroid function by the hypothalamus and anterior pituitary
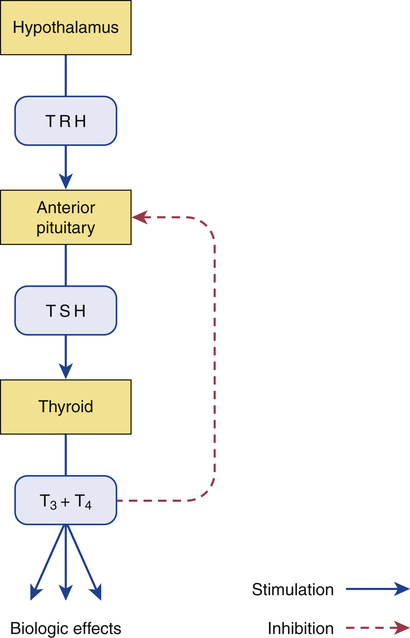
 Regulation of thyroid function.
Regulation of thyroid function.
TRH from the hypothalamus stimulates release of TSH from the pituitary. TSH stimulates all aspects of thyroid function, including release of T3 and T4. T3 and T4 act on the pituitary to suppress further TSH release. (T3 = triiodothyronine, T4 = thyroxine, TRH = thyrotropin-releasing hormone, TSH = thyrotropin [thyroid-stimulating hormone].)
Thyroid function tests

Serum Values
Thyroid Test
Normal
Hypothyroid
Hyperthyroid
Total T4 (mcg/dL)
4.5–12.5
Under 4.5
Over 12.5
Free T4 (ng/dL)
0.9–2
Under 0.9
Over 2
Total T3 (ng/dL)
80–220
Under 80
Over 220
Free T3 (pg/dL)
230–620
Under 230
Over 620
TSH (microunits/mL)
0.3–6
Over 6
Under 0.3
Thyroid pathophysiology
Hypothyroidism
Hypothyroidism during pregnancy
Thyroid hormone preparations for hypothyroidism

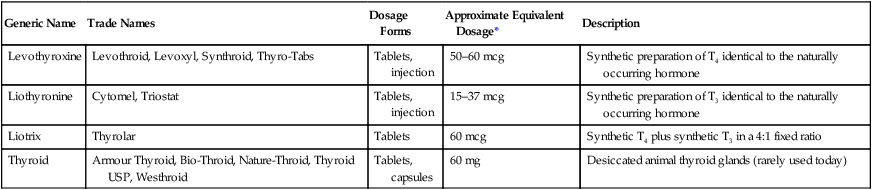
Generic Name
Trade Names
Dosage Forms
Approximate Equivalent Dosage*
Description
Levothyroxine
Levothroid, Levoxyl, Synthroid, Thyro-Tabs
Tablets, injection
50–60 mcg
Synthetic preparation of T4 identical to the naturally occurring hormone
Liothyronine
Cytomel, Triostat
Tablets, injection
15–37 mcg
Synthetic preparation of T3 identical to the naturally occurring hormone
Liotrix
Thyrolar
Tablets
60 mcg
Synthetic T4 plus synthetic T3 in a 4:1 fixed ratio
Thyroid
Armour Thyroid, Bio-Throid, Nature-Throid, Thyroid USP, Westhroid
Tablets, capsules
60 mg
Desiccated animal thyroid glands (rarely used today)
Drugs for thyroid disorders
Get Clinical Tree app for offline access



