CHAPTER 105 The skin is composed of three distinct layers: the epidermis, the dermis, and a layer of subcutaneous fat. These layers and other features of the skin are depicted in Figure 105–1. The epidermis is the outermost layer of the skin and is composed almost entirely of closely packed cells. As indicated in Figure 105–1B, the epidermis itself consists of several layers. The deepest, known as the basal layer or stratum germinativum, contains the only epidermal cells that are mitotically active. All cells of the epidermis arise from this layer. Production of new cells within the basal layer pushes older cells outward. During their migration, these cells become smaller and flatter. As epidermal cells near the surface of the skin, they die and their cytoplasm is converted to keratin, a hard, proteinaceous material. Because of its high content of keratin, the outer layer of the epidermis has a rough, horny texture. Because of its texture, this layer is referred to as the cornified layer or stratum corneum. By a process that is not fully understood, the surface of the stratum corneum undergoes continuous exfoliation (shedding). This shedding completes the epidermal growth cycle. The dermis underlies the epidermis and is composed largely of connective tissue, primarily collagen. A major function of the dermis is to provide support and nourishment for the epidermis. Structures found in the dermis include blood vessels, nerves, and muscle. The dermis also contains sweat glands, sebaceous glands, and hair follicles. Sebaceous glands secrete an oily composite known as sebum. Almost all sebaceous glands are associated with hair follicles (see Fig. 105–1A). The basic pharmacology of the glucocorticoids (anti-inflammatory corticosteroids) is discussed in Chapter 72. Consideration here is limited to their use for skin disorders. Topical glucocorticoids are employed to relieve inflammation and itching associated with a variety of dermatologic conditions (eg, insect bites, minor burns, seborrheic dermatitis, psoriasis, eczema, pemphigus). The mechanisms by which glucocorticoids suppress inflammation and other symptoms are discussed in Chapter 72. Glucocorticoid preparations vary widely in potency. As indicated in Table 105–1, these drugs can be assigned to four groups that range in potency from low to super high. Preparations within each group are equipotent. TABLE 105–1 Relative Potency of Topical Glucocorticoids Topical glucocorticoids can be absorbed in amounts sufficient to produce systemic toxicity. Principal concerns are growth retardation (in children) and adrenal suppression (in all age groups). Systemic toxicity is more likely under extreme conditions of use (prolonged therapy in which a large area is treated with big doses of a high-potency agent covered with an occlusive dressing). When these conditions are present, the hypothalamic-pituitary-adrenal axis should be monitored. Systemic toxicity of the glucocorticoids is discussed at length in Chapter 72. Drugs for acne fall into two major groups: topical drugs and oral drugs (Table 105–2). The topical drugs have two principal subgroups: antimicrobial agents and retinoids. Likewise, the oral drugs have two principal subgroups: antibiotics and retinoids. TABLE 105–2 iPLEDGE is the name of a very strict risk management program designed to ensure that no woman starting isotretinoin is pregnant and that no woman taking isotretinoin becomes pregnant. The iPLEDGE program, which went into effect December 31, 2005, replaced S.M.A.R.T. (System to Manage Accutane-Related Teratogenicity) and all other programs designed to guard against use of isotretinoin during pregnancy. The principal difference between iPLEDGE and S.M.A.R.T. is that, under iPLEDGE, all transactions involving isotretinoin must be processed through a central automated system, which tracks and verifies critical elements that control access to the drug. The program has rules that apply to the prescriber, patient, pharmacist, and wholesaler. Details regarding iPLEDGE are available online at www.ipledgeprogram.com. Spironolactone [Aldactone] blocks a variety of steroid receptors, including those for aldosterone and sex hormones. Blockade of aldosterone receptors underlies the drug’s use as a diuretic (see Chapter 41) as well as its use in heart failure (see Chapter 48). Blockade of androgen receptors underlies benefits in females with acne. As a rule, spironolactone is added to the regimen after an oral contraceptive has proved inadequate. This sequence makes sense. Why? Because spironolactone is teratogenic, and hence contraception should be implemented before taking the drug. Adverse effects include menstrual irregularities, breast tenderness, and hyperkalemia.
Drugs for the skin
Anatomy of the skin
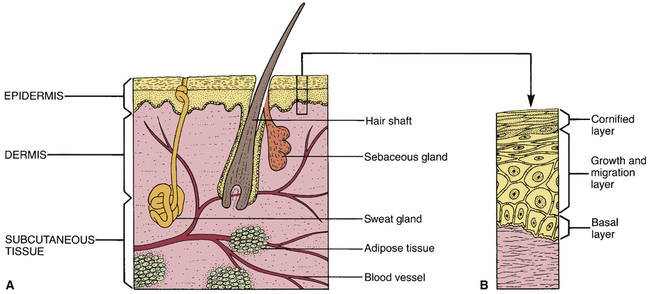
 Anatomy of the skin.
Anatomy of the skin.
A, Major structures of the skin. B, Growth layers of the epidermis.
Epidermis.
Dermis.
Topical glucocorticoids
Actions and uses.
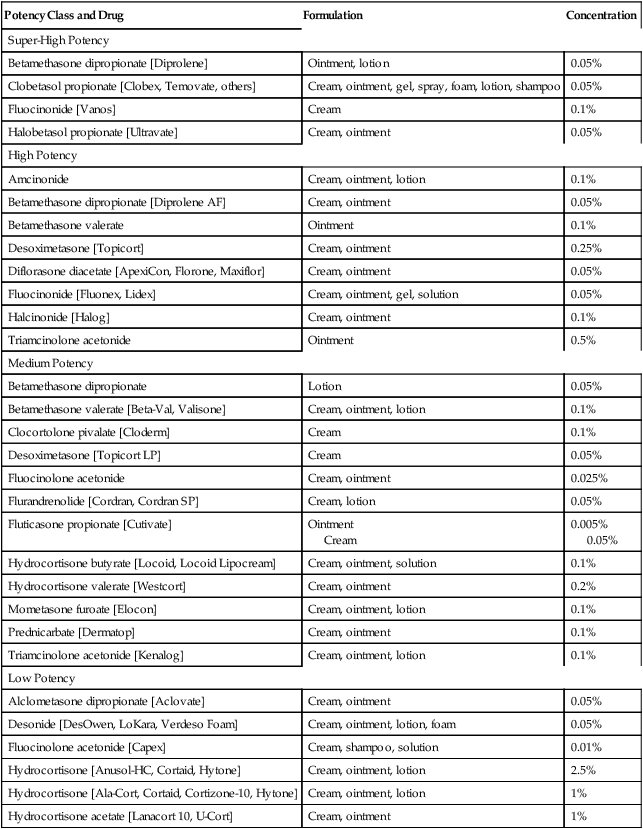
Relative potency.

Potency Class and Drug
Formulation
Concentration
Super-High Potency
Betamethasone dipropionate [Diprolene]
Ointment, lotion
0.05%
Clobetasol propionate [Clobex, Temovate, others]
Cream, ointment, gel, spray, foam, lotion, shampoo
0.05%
Fluocinonide [Vanos]
Cream
0.1%
Halobetasol propionate [Ultravate]
Cream, ointment
0.05%
High Potency
Amcinonide
Cream, ointment, lotion
0.1%
Betamethasone dipropionate [Diprolene AF]
Cream, ointment
0.05%
Betamethasone valerate
Ointment
0.1%
Desoximetasone [Topicort]
Cream, ointment
0.25%
Diflorasone diacetate [ApexiCon, Florone, Maxiflor]
Cream, ointment
0.05%
Fluocinonide [Fluonex, Lidex]
Cream, ointment, gel, solution
0.05%
Halcinonide [Halog]
Cream, ointment
0.1%
Triamcinolone acetonide
Ointment
0.5%
Medium Potency
Betamethasone dipropionate
Lotion
0.05%
Betamethasone valerate [Beta-Val, Valisone]
Cream, ointment, lotion
0.1%
Clocortolone pivalate [Cloderm]
Cream
0.1%
Desoximetasone [Topicort LP]
Cream
0.05%
Fluocinolone acetonide
Cream, ointment
0.025%
Flurandrenolide [Cordran, Cordran SP]
Cream, lotion
0.05%
Fluticasone propionate [Cutivate]
Ointment
Cream
0.005%
0.05%
Hydrocortisone butyrate [Locoid, Locoid Lipocream]
Cream, ointment, solution
0.1%
Hydrocortisone valerate [Westcort]
Cream, ointment
0.2%
Mometasone furoate [Elocon]
Cream, ointment, lotion
0.1%
Prednicarbate [Dermatop]
Cream, ointment
0.1%
Triamcinolone acetonide [Kenalog]
Cream, ointment, lotion
0.1%
Low Potency
Alclometasone dipropionate [Aclovate]
Cream, ointment
0.05%
Desonide [DesOwen, LoKara, Verdeso Foam]
Cream, ointment, lotion, foam
0.05%
Fluocinolone acetonide [Capex]
Cream, shampoo, solution
0.01%
Hydrocortisone [Anusol-HC, Cortaid, Hytone]
Cream, ointment, lotion
2.5%
Hydrocortisone [Ala-Cort, Cortaid, Cortizone-10, Hytone]
Cream, ointment, lotion
1%
Hydrocortisone acetate [Lanacort 10, U-Cort]
Cream, ointment
1%
Adverse effects.
Acne
Overview of treatment
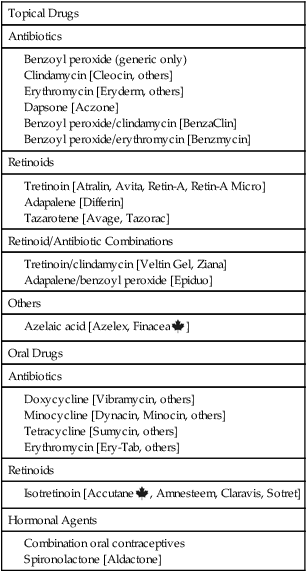
Drug therapy

Topical Drugs
Antibiotics
Retinoids
Retinoid/Antibiotic Combinations
Others
Oral Drugs
Antibiotics
Retinoids
Hormonal Agents
Oral drugs for acne
Isotretinoin
IPLEDGE program.
Hormonal agents
Spironolactone.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drugs for the skin
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access
