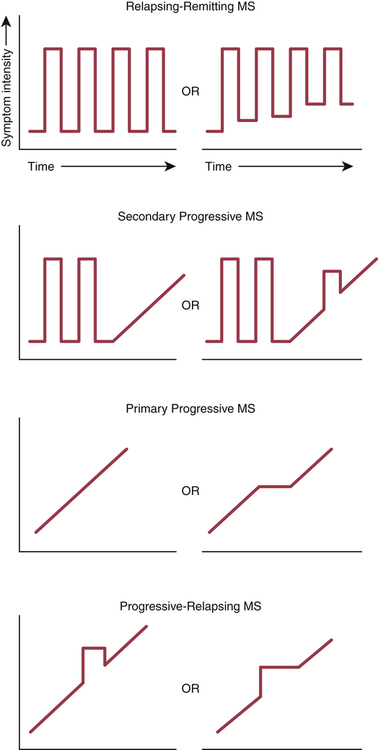
CHAPTER 23 There are four subtypes of MS—relapsing-remitting, secondary progressive, primary progressive, and progressive-relapsing—defined by the clinical course the disease follows. Symptom patterns that characterize the MS subtypes are depicted in Figure 23–1. In 1965, the following diagnostic criteria were introduced: • There must be objective evidence of two or more clinical attacks lasting at least 24 hours each. • The attacks must be separated in time by at least 1 month. • The attacks must be separated in space (that is, the CNS damage underlying clinical symptoms must occur at different sites). • MRI is the most sensitive way to image the brain. Sensitivity can be made even greater with gadolinium, an IV contrast agent. MRI is especially good for detecting areas of demyelination. However, it is important to note that, in some patients who have clinically definite MS, an MRI scan may fail to detect any lesions. Hence, a negative scan does not necessarily rule out MS. Conversely, because other disorders can produce a positive scan, a positive scan, by itself, does not prove the presence of MS, although it can help confirm a suspected case. • Tests of CSF are used to assess immune activity within the CNS. Two tests are employed: measurement of immunoglobulin G (IgG) levels and measurement of oligoclonal IgG bands (OCBs), which indicate intrathecal production of antibodies. More than 90% of patients with MS have OCBs. However, as with MRI scans, other disorders can also produce positive test results—and negative results may be seen in some patients who do have MS. Accordingly, CSF analysis, by itself, can neither confirm nor rule out MS. • The VEP test measures how quickly the brain responds to a visual stimulus, and hence indirectly measures conduction velocity in the optic nerve. In patients with MS, the VEP is delayed, indicating conduction velocity is slowed (owing to demyelination of the optic nerve). However, as with MRI scans and CSF tests, the VEP test is not specific for MS: Other disorders can produce positive results, and some patients with MS may get negative results. Diagnostic criteria for MS were revised by the McDonald committee in 2001, revised again in 2005, and revised yet again in 2010 (Table 23–1). The latest criteria are still founded on the patient’s clinical presentation, but also incorporate information from MRI scans and CSF tests. These criteria were adopted to permit the earliest possible diagnosis of MS, and hence permit the earliest possible implementation of disease-modifying therapy. TABLE 23–1 2010 Revised McDonald Criteria for Diagnosis of MS *CSF = cerebrospinal fluid. A positive CSF test shows either oligoclonal bands different from those in serum or a raised IgG index. Adapted from Polman CH, Reingold SC, Banwell B, et al: Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald Criteria. Ann Neurol 69:292–302, 2011. There are two main groups of disease-modifying drugs: immunomodulators and immunosuppressants (Table 23–2). The immunomodulators—interferon beta, glatiramer acetate, natalizumab, and fingolimod—are safer than mitoxantrone (the major immunosuppressant in use), and hence are generally preferred. TABLE 23–2 Disease-Modifying Drugs for MS *Maximum lifetime dose is 140 mg/m2 (because of cardiotoxicity). A short course of a high-dose IV glucocorticoid (eg, 500 mg to 1 gm of methylprednisolone daily for 3 to 5 days) is the preferred treatment of an acute relapse. Glucocorticoids suppress inflammation and can thereby reduce the severity and duration of a clinical attack. As discussed in Chapter 72, these drugs are very safe when used short term, elevation of blood glucose being the principal concern. By contrast, long-term exposure can cause osteoporosis and other serious adverse effects. Accordingly, frequent use (more than 3 times a year) or prolonged use (longer than 3 weeks at a time) should be avoided. With the exception of fingolimod, all of the first-line immunomodulators are administered by self-injection (IM or SQ). Fingolimod is administered PO. Natalizumab, a second-line drug, is administered by IV infusion (at an approved infusion center). All seven drugs are expensive: One year of treatment costs between $35,000 and $48,000. Properties of these drugs are summarized in Table 23–2.
Drugs for multiple sclerosis
Overview of MS and its treatment
Pathophysiology
MS subtypes
Diagnosis

Number of Attacks
Clinical Evidence of Lesions
Additional Data Needed for a Diagnosis of MS
2 or more
Objective clinical evidence of 2 or more lesions or objective clinical evidence of 1 lesion with reasonable historic evidence of a prior attack
None: Clinical evidence alone will suffice.
2 or more
Objective clinical evidence of 1 lesion
Proof of dissemination in space shown by either MRI (ie, at least 2 lesions visible on the MRI in CNS regions typically affected by MS) or await another clinical attack implicating a different CNS site.
1
Objective clinical evidence of 2 or more lesions
Proof of dissemination in time shown by either MRI (eg, a new lesion is visible on a follow-up MRI) or await a second clinical attack
1
Objective clinical evidence of 1 lesion (clinically isolated syndrome)
Proof of dissemination in space shown by either MRI or await a second clinical attack implicating a different site
plus
Proof of dissemination in time shown by either MRI or await a second clinical attack
0 (Insidious neurologic progression suggestive of MS but with no clear clinical attack)
One year of disease progression (retrospectively or prospectively determined)
plus
Two out of three of the following:

Drug therapy overview
Disease-modifying therapy

Generic Name
Trade Name
Route
Dose
Dosing Schedule
Annual Cost
Adverse Effects
IMMUNOMODULATORS
Interferon Beta Preparations
Interferon beta-1a
Avonex
IM
30 mcg
Once a week
$35,600
All three preparations:
Interferon beta-1a
Interferon beta-1b
Rebif
Betaseron, Extavia
subQ
subQ
44 mcg
250 mcg
3 times a week
Every other day
$35,700
$35,400
Other Immunomodulators
Glatiramer acetate
Copaxone
subQ
20 mg
Once a day
$43,000
Natalizumab
Tysabri
IV
300 mg
Every 4 weeks
$40,400
Fingolimod
Gilenya
PO
0.5 mg
Once a day
$48,000
IMMUNOSUPPRESSANT
Mitoxantrone
Novantrone
IV
12 mg/m2*
Every 3 months
$6,300

Treating an acute episode (relapse)
Disease-modifying drugs I: immunomodulators
Drugs for multiple sclerosis
Get Clinical Tree app for offline access