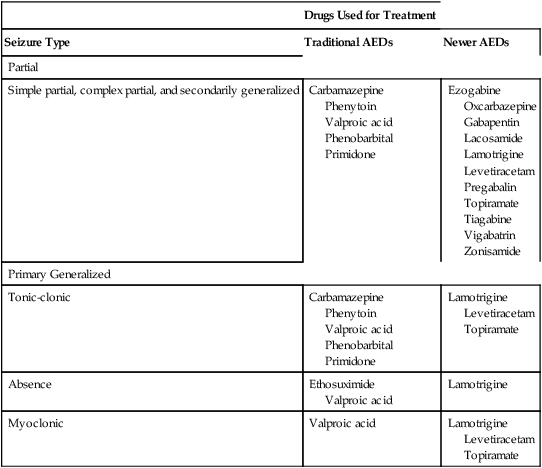
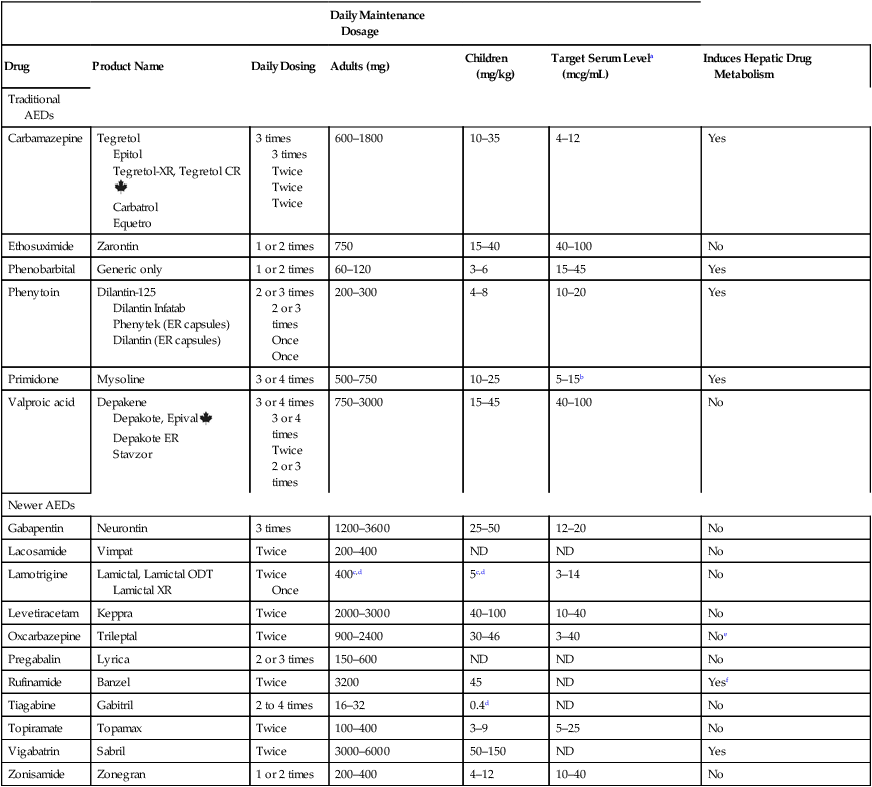
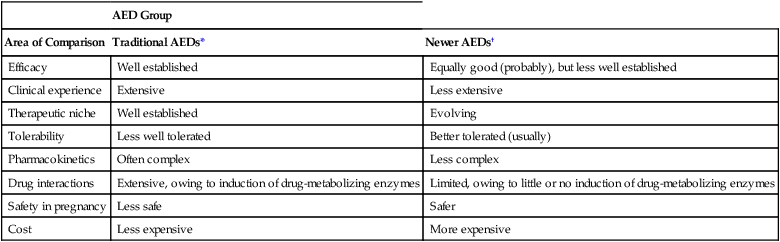
CHAPTER 24 Seizure can be divided into two broad categories: partial (focal) seizures and generalized seizures. In partial seizures, seizure activity begins focally in the cerebral cortex and usually undergoes limited spread to adjacent cortical areas. In generalized seizures, focal seizure activity is conducted widely throughout both hemispheres. As a rule, partial seizures and generalized seizures are treated with different drugs (Table 24–1). TABLE 24–1 Drugs for Specific Types of Seizures Epilepsy may be treated with drugs or with nondrug therapies. As noted, drugs can benefit 60% to 70% of patients. This means that, of the 2.3 million Americans with epilepsy, between 690,000 and 920,000 cannot be treated successfully with drugs. For these people, nondrug therapy may well help. Three options exist: neurosurgery, vagus nerve stimulation, and the ketogenic diet. Of the three, neurosurgery has the best success rate, but vagus nerve stimulation is used most widely. All three nondrug therapies are discussed in Box 24–1. Control of seizures requires proper drug selection. As indicated in Table 24–1, many AEDs are selective for specific seizure disorders. Phenytoin, for example, is useful for treating tonic-clonic and partial seizures but not absence seizures. Conversely, ethosuximide is active against absence seizures but not against tonic-clonic or partial seizures. Only one drug—valproic acid—appears effective against practically all forms of epilepsy. Since most AEDs are selective for certain seizure disorders, effective treatment requires a proper match between the drug and the seizure. To make this match, the seizure type must be accurately diagnosed. Monitoring plasma levels of AEDs is common. Safe and effective levels have been firmly established for most AEDs (Table 24–2). Monitoring these levels can help guide dosage adjustments. TABLE 24–2 Clinical Pharmacology of the Oral Antiepileptic Drugs (AEDs) bTarget serum level is 5 to 15 mcg/mL for primidone itself, and 15 to 40 mcg/mL for phenobarbital derived from primidone. cDosage must be decreased in patients taking valproic acid. dDosage must be increased in patients taking drugs that induce hepatic drug-metabolizing enzymes. eOxcarbazepine does not induce enzymes that metabolize AEDs, but does induce enzymes that metabolize other drugs. The AEDs can be grouped into two major categories: traditional AEDs and newer AEDs. The traditional group has six major members, the last of which—valproic acid—was approved in 1978. The group of newer AEDs has thirteen members, all of which were approved in 1993 or later. As summarized in Table 24–3, both groups have their advantages and disadvantages. For example, clinical experience with the older AEDs is more extensive than with the newer ones, and the older drugs cost less. Both facts make the older drugs attractive. However, the older AEDs also have drawbacks, including troublesome side effects and complex drug interactions. Of importance, drugs in both groups appear equally effective—although few direct comparisons have been made. The bottom line? Neither group is clearly superior to the other. Hence, when selecting an AED, drugs in both groups should be considered. TABLE 24–3 Comparison of Traditional and Newer Antiepileptic Drugs *Carbamazepine, ethosuximide, phenobarbital, phenytoin, primidone, and valproic acid. †Gabapentin, lacosamide, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, rufinamide, tiagabine, topiramate, vigabatrin, and zonisamide. The capacity of the liver to metabolize phenytoin is very limited. As a result, the relationship between dosage and plasma levels of phenytoin is unusual. Doses of phenytoin needed to produce therapeutic effects are only slightly smaller than the doses needed to saturate the hepatic enzymes that metabolize phenytoin. Consequently, if phenytoin is administered in doses only slightly greater than those needed for therapeutic effects, the liver’s capacity to metabolize the drug will be overwhelmed, causing plasma levels of phenytoin to rise dramatically. This unusual relationship between dosage and plasma levels is illustrated in Figure 24–1A. As you can see, once plasma levels have reached the therapeutic range, small changes in dosage produce large changes in plasma levels. As a result, small increases in dosage can cause toxicity, and small decreases can cause therapeutic failure. This relationship makes it difficult to establish and maintain a dosage that is both safe and effective. Figure 24–1B indicates the relationship between dosage and plasma levels that exists for most drugs. As indicated, this relationship is linear, in contrast to the nonlinear relationship that exists for phenytoin. Accordingly, for most drugs, if the patient is taking doses that produce plasma levels that are within the therapeutic range, small deviations from that dosage produce only small deviations in plasma drug levels. Because of this relationship, with most drugs it is relatively easy to maintain plasma levels that are safe and effective.
Drugs for epilepsy
Types of seizures

Drugs Used for Treatment
Seizure Type
Traditional AEDs
Newer AEDs
Partial
Simple partial, complex partial, and secondarily generalized
Carbamazepine
Phenytoin
Valproic acid
Phenobarbital
Primidone
Ezogabine
Oxcarbazepine
Gabapentin
Lacosamide
Lamotrigine
Levetiracetam
Pregabalin
Topiramate
Tiagabine
Vigabatrin
Zonisamide
Primary Generalized
Tonic-clonic
Carbamazepine
Phenytoin
Valproic acid
Phenobarbital
Primidone
Lamotrigine
Levetiracetam
Topiramate
Absence
Ethosuximide
Valproic acid
Lamotrigine
Myoclonic
Valproic acid
Lamotrigine
Levetiracetam
Topiramate
Basic therapeutic considerations
Therapeutic goal and treatment options
Diagnosis and drug selection
Monitoring plasma drug levels

Daily Maintenance Dosage
Drug
Product Name
Daily Dosing
Adults (mg)
Children (mg/kg)
Target Serum Levela (mcg/mL)
Induces Hepatic Drug Metabolism
Traditional AEDs
Carbamazepine
Tegretol
Epitol
Tegretol-XR, Tegretol CR![]()
Carbatrol
Equetro
3 times
3 times
Twice
Twice
Twice
600–1800
10–35
4–12
Yes
Ethosuximide
Zarontin
1 or 2 times
750
15–40
40–100
No
Phenobarbital
Generic only
1 or 2 times
60–120
3–6
15–45
Yes
Phenytoin
Dilantin-125
Dilantin Infatab
Phenytek (ER capsules)
Dilantin (ER capsules)
2 or 3 times
2 or 3 times
Once
Once
200–300
4–8
10–20
Yes
Primidone
Mysoline
3 or 4 times
500–750
10–25
5–15b
Yes
Valproic acid
Depakene
Depakote, Epival![]()
Depakote ER
Stavzor
3 or 4 times
3 or 4 times
Twice
2 or 3 times
750–3000
15–45
40–100
No
Newer AEDs
Gabapentin
Neurontin
3 times
1200–3600
25–50
12–20
No
Lacosamide
Vimpat
Twice
200–400
ND
ND
No
Lamotrigine
Lamictal, Lamictal ODT
Lamictal XR
Twice
Once
400c,d
5c,d
3–14
No
Levetiracetam
Keppra
Twice
2000–3000
40–100
10–40
No
Oxcarbazepine
Trileptal
Twice
900–2400
30–46
3–40
Noe
Pregabalin
Lyrica
2 or 3 times
150–600
ND
ND
No
Rufinamide
Banzel
Twice
3200
45
ND
Yesf
Tiagabine
Gabitril
2 to 4 times
16–32
0.4d
ND
No
Topiramate
Topamax
Twice
100–400
3–9
5–25
No
Vigabatrin
Sabril
Twice
3000–6000
50–150
ND
Yes
Zonisamide
Zonegran
1 or 2 times
200–400
4–12
10–40
No
Classification of antiepileptic drugs

AED Group
Area of Comparison
Traditional AEDs*
Newer AEDs†
Efficacy
Well established
Equally good (probably), but less well established
Clinical experience
Extensive
Less extensive
Therapeutic niche
Well established
Evolving
Tolerability
Less well tolerated
Better tolerated (usually)
Pharmacokinetics
Often complex
Less complex
Drug interactions
Extensive, owing to induction of drug-metabolizing enzymes
Limited, owing to little or no induction of drug-metabolizing enzymes
Safety in pregnancy
Less safe
Safer
Cost
Less expensive
More expensive
Traditional antiepileptic drugs
Phenytoin
Pharmacokinetics
Metabolism.
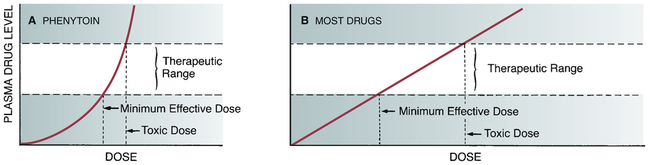
 Relationship between dose and plasma level for phenytoin compared with most other drugs.
Relationship between dose and plasma level for phenytoin compared with most other drugs.
A, Within the therapeutic range, small increments in phenytoin dosage produce sharp increases in plasma drug levels. This relationship makes it difficult to maintain plasma phenytoin levels within the therapeutic range. B, Within the therapeutic range, small increments in dosage of most drugs produce small increases in drug levels. With this relationship, moderate fluctuations in dosage are unlikely to result in either toxicity or therapeutic failure.




