CHAPTER 57 Marshal Shlafer and Rich Lehne There are two main forms of diabetes mellitus: type 1 diabetes mellitus and type 2 diabetes mellitus. Both forms have similar signs and symptoms. Major differences concern etiology, prevalence, treatments, and outcomes (illness severity and deaths). The distinguishing characteristics of type 1 and type 2 diabetes are summarized in Table 57–1 and discussed immediately below. Another important form—gestational diabetes—is discussed later under Diabetes and Pregnancy. TABLE 57–1 Characteristics of Type 1 and Type 2 Diabetes Mellitus Treatment of diabetes can delay the onset of nephropathy and reduce its severity. The Diabetes Control and Complications Trial (DCCT) revealed that tight glucose control decreases the risk of nephropathy by 35% to 57%. As discussed in Chapter 44, treatment with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB) can slow progression of mild-to-moderate nephropathy that is already present. However, these drugs are not effective for primary prevention. Of note, ACE inhibitors and ARBs have an additional benefit: they can help control hypertension, a common complication of diabetes. Diabetic gastroparesis (delayed stomach emptying) affects 20% to 30% of patients with long-standing diabetes. Manifestations include nausea, vomiting, delayed gastric emptying, and gastric or intestinal distention. Injury to the autonomic nerves that control GI motility seems to be the underlying cause. Symptoms can be reduced with metoclopramide [Reglan], a dopamine antagonist that promotes gastric emptying (see Chapter 80). In addition to affecting autonomic nerves that innervate the GI tract, diabetes can affect autonomic nerves that innervate other structures. Until recently, diagnosis of diabetes was made solely on measuring blood levels of glucose. However, in 2010, the American Diabetes Association (ADA) recommended an alternative test, based on measuring blood levels of a compound known as hemoglobin A1c. For all of these tests, values diagnostic of diabetes are summarized in Table 57–2. TABLE 57–2 Criteria for the Diagnosis of Diabetes Mellitus Fasting plasma glucose ≥126 mg/dL* or Casual plasma glucose ≥200 mg/dL plus symptoms of diabetes† or Oral glucose tolerance test (OGTT): 2-hr plasma glucose ≥200 mg/dL‡ or Hemoglobin A1c 6.5% or higher *Fasting is defined as no caloric intake for at least 8 hours. †Casual is defined as any time of day without regard to meals. Classic symptoms of diabetes include polyuria, polydipsia, and unexplained weight loss. ‡In this OGTT, plasma glucose content is measured 2 hours after ingesting the equivalent of 75 gm of anhydrous glucose dissolved in water. The OGTT is not recommended or needed for routine clinical use. Adapted from Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 26(Suppl 1):S5–S24, 2003. The primary goal of treating type 1 or type 2 diabetes is prevention of long-term complications, especially CVD, retinopathy, kidney disease, and amputations. To minimize these complications, treatment must keep glucose levels as low as safely possible. In addition, treatment must keep blood pressure and blood lipids within an acceptable range. In both type 1 and type 2 diabetes, proper diet and adequate exercise are central components of management. Major treatment targets are summarized in Table 57–3. TABLE 57–3 General Treatment Targets for Patients with Diabetes* *Treatment targets for certain subgroups of patients may differ from the targets in this table. †An albumin/creatinine ratio above 30 mcg albumin/1 mg creatinine indicates too much albumin in urine owing to glomerular injury. • Carbohydrates and monounsaturated fats, together, should provide 60% to 70% of daily energy intake. • Protein should provide 15% to 20% of daily energy intake. • Polyunsaturated fat should provide about 10% of daily energy intake. • Saturated fats should provide less than 10% of daily energy intake. • Cholesterol intake should be limited to 300 mcg/day. • Total caloric intake should be spread evenly throughout the day, with meals spaced 4 to 5 hours apart. As noted earlier, an ACE inhibitor (eg, captopril) or an ARB (eg, losartan) can reduce the risk of diabetic nephropathy, a long-term consequence of poor glycemic control. These same drugs are preferred agents for managing diabetic hypertension. As indicated in Table 57–3, the goal is to keep blood pressure at or below 130/80 mm Hg. Step 1. At diagnosis, initiate lifestyle changes plus metformin. Step 2. Continue lifestyle changes plus metformin, and add a second drug, either basal insulin or a sulfonylurea. Step 3. Continue lifestyle changes plus metformin, and switch from basal insulin or a sulfonylurea to intensive insulin therapy. The effects of tight glycemic control in type 2 diabetes were demonstrated in four landmark trials: • United Kingdom Prospective Diabetes Study (UKPDS) • Action to Control Cardiovascular Risk in Diabetes (ACCORD) • Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) • Long-standing type 2 diabetes • Advanced microvascular or macrovascular complications • Extensive comorbid conditions • A history of severe hypoglycemia For these patients, an A1c goal above 7% may be more appropriate than a goal below 7%. We need monitoring to (1) determine whether glucose levels are being maintained in a safe range, both short term and long term, and to (2) guide changes in the regimen when the range is not satisfactory or safe. Self-measurement of blood glucose levels is the standard method for day-to-day monitoring. A1c is measured to assess long-term glycemic control. Target levels for these tests are summarized in Table 57–4. TABLE 57–4 Hemoglobin A1c Levels and Their Corresponding eAG Levels* eAG = estimated Average Glucose in blood. *The formula to convert from A1c (%) to average glucose concentration equivalents (expressed in mg/dL) is: eAG = (A1c × 28.7) − 46.7. †7% A1c (corresponding to an eAG of 154 mg/dL) is considered to be the maximum acceptable level for long-term glycemic control. For many patients, achieving this degree of control is difficult or impossible. Data from American Diabetes Association. How are test results expressed? Results are usually reported as a percent of total hemoglobin in blood (eg, 7%). In addition, they may be reported as a value for estimated Average Glucose (eAG), expressed as mg glucose/dL of blood (ie, the same units patients see every day when doing SMBG). Selected A1c values and their eAG equivalents are listed in Table 57–4. The structure of insulin is depicted in Figure 57–1. As indicated, insulin consists of two amino acid chains: the “A” (acidic) chain and the “B” (basic) chain. The A and B chains are linked to each other by two disulfide bridges. Insulin acts in two ways to promote anabolic effects. First, it stimulates cellular transport (uptake) of glucose, amino acids, nucleotides, and potassium. Second, insulin promotes synthesis of complex organic molecules. Under the influence of insulin and other factors, glucose is converted into glycogen (the liver’s way to store glucose for later use), amino acids are assembled into proteins, and fatty acids are incorporated into triglycerides. The principal metabolic actions of insulin are summarized in Table 57–5. TABLE 57–5 *Because of decreased delivery of substrate (fatty acids and amino acids) to the liver. There are seven types of insulin: “natural” insulin (also known as regular insulin or native insulin) and six modified insulins. Three of the modified insulins—insulin lispro, insulin aspart, and insulin glulisine—act more rapidly than natural insulin but have a shorter duration of action. The other modified insulins act more slowly than natural insulin but have a longer duration. Two processes are used to prolong insulin effects: (1) complexing natural insulin with a protein, and (2) altering the insulin molecule itself. When the insulin molecule has been altered, we refer to the product as a human insulin analog. Specific alterations made to create the insulin analogs are summarized in Table 57–6. TABLE 57–6 Amino Acids Substitutions in Human Insulin Analogs*
Drugs for diabetes mellitus
Diabetes mellitus: basic considerations
Types of diabetes mellitus

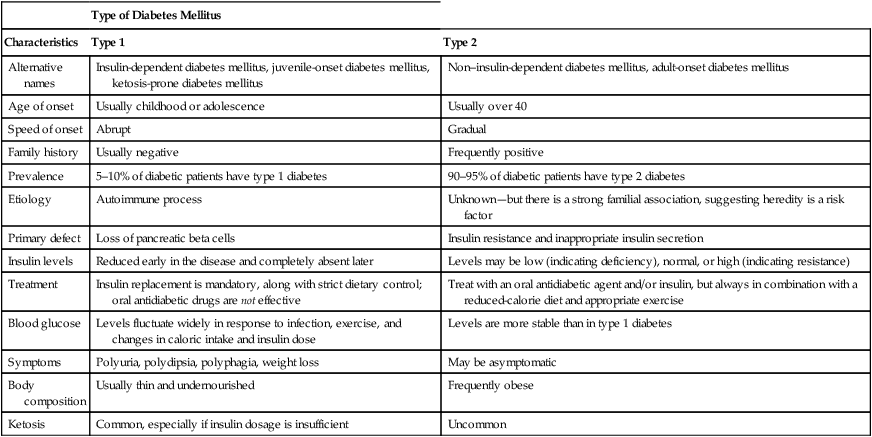
Type of Diabetes Mellitus
Characteristics
Type 1
Type 2
Alternative names
Insulin-dependent diabetes mellitus, juvenile-onset diabetes mellitus, ketosis-prone diabetes mellitus
Non–insulin-dependent diabetes mellitus, adult-onset diabetes mellitus
Age of onset
Usually childhood or adolescence
Usually over 40
Speed of onset
Abrupt
Gradual
Family history
Usually negative
Frequently positive
Prevalence
5–10% of diabetic patients have type 1 diabetes
90–95% of diabetic patients have type 2 diabetes
Etiology
Autoimmune process
Unknown—but there is a strong familial association, suggesting heredity is a risk factor
Primary defect
Loss of pancreatic beta cells
Insulin resistance and inappropriate insulin secretion
Insulin levels
Reduced early in the disease and completely absent later
Levels may be low (indicating deficiency), normal, or high (indicating resistance)
Treatment
Insulin replacement is mandatory, along with strict dietary control; oral antidiabetic drugs are not effective
Treat with an oral antidiabetic agent and/or insulin, but always in combination with a reduced-calorie diet and appropriate exercise
Blood glucose
Levels fluctuate widely in response to infection, exercise, and changes in caloric intake and insulin dose
Levels are more stable than in type 1 diabetes
Symptoms
Polyuria, polydipsia, polyphagia, weight loss
May be asymptomatic
Body composition
Usually thin and undernourished
Frequently obese
Ketosis
Common, especially if insulin dosage is insufficient
Uncommon
Long-term complications of diabetes
Microvascular damage
Nephropathy.
Autonomic neuropathy: gastroparesis.
Diagnosis

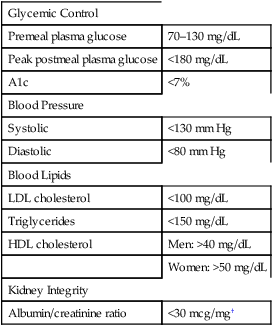
Overview of treatment

Glycemic Control
Premeal plasma glucose
70–130 mg/dL
Peak postmeal plasma glucose
<180 mg/dL
A1c
<7%
Blood Pressure
Systolic
<130 mm Hg
Diastolic
<80 mm Hg
Blood Lipids
LDL cholesterol
<100 mg/dL
Triglycerides
<150 mg/dL
HDL cholesterol
Men: >40 mg/dL
Women: >50 mg/dL
Kidney Integrity
Albumin/creatinine ratio
<30 mcg/mg†
Type 1 diabetes
Dietary measures.
Managing hypertension and dyslipidemia.
Type 2 diabetes
Tight glycemic control
Type 2 diabetes
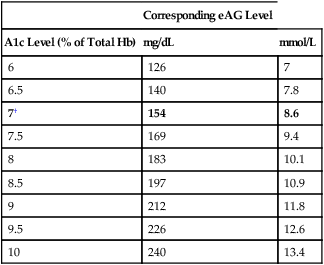
Monitoring treatment

Corresponding eAG Level
A1c Level (% of Total Hb)
mg/dL
mmol/L
6
126
7
6.5
140
7.8
7†
154
8.6
7.5
169
9.4
8
183
10.1
8.5
197
10.9
9
212
11.8
9.5
226
12.6
10
240
13.4
Monitoring of hemoglobin A1C
Insulin
Physiology
Structure
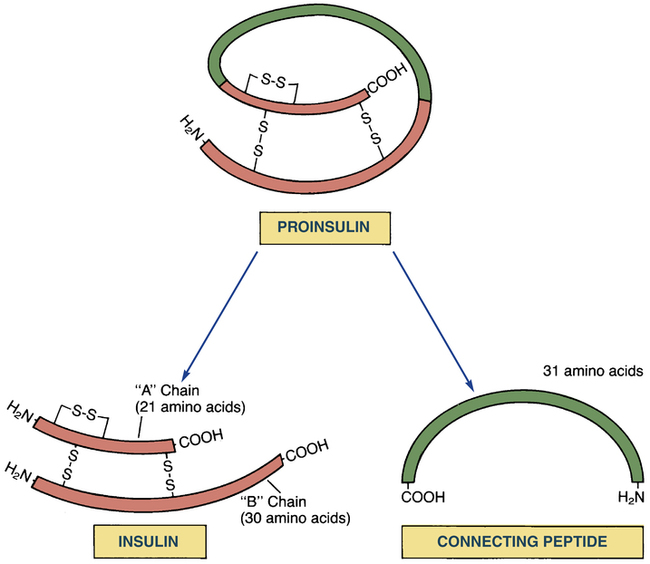
 Conversion of proinsulin to insulin.
Conversion of proinsulin to insulin.
Proinsulin is the immediate precursor of the insulin secreted by our pancreas. Enzymes clip off connecting peptide (C-peptide) to release active insulin, composed of two peptide chains (A and B) connected by two disulfide (S–S) bonds. Since C-peptide arises only from endogenous insulin, its presence in blood indicates that at least some pancreatic insulin is being made.
Metabolic actions

Substance Affected
Insulin Action
Site of Action
Carbohydrates
↑ Glucose uptake
↑ Glucose oxidation
↑ Glucose storage
↑ Glycogen synthesis
↓ Glycogenolysis
Gluconeogenesis*
Muscle, adipose tissue
Muscle
Muscle, liver
Liver
Amino Acids and Proteins
↑ Amino acid uptake
↓ Amino acid release
↑ Protein synthesis
Muscle
Muscle
Muscle
Lipids
↑ Triglyceride synthesis
↓ Release of FFA† and glycerol
↓ Oxidation of FFA to ketoacids‡
Adipose tissue
Adipose tissue
Liver
Preparations and administration
Types of insulin

Insulin Type
Amino Acids in A-Chain Position
Amino Acids in B-Chain Position
A8
A10
A21
B3
B28
B29
B30
B31
B32
Human Insulin
Native†
Thr
Ilc
Asn
Asn
Pro
Lys
Thr
—
—
Human Insulin Analogs
Glargine
Thr
Ilc
Gly
Gly
Pro
Lys
Thr
Arg
Arg
Aspart
Thr
Ilc
Asn
Asn
Asp
Lys
Thr
—
—
Lispro
Thr
Ilc
Asn
Asn
Lys
Pro
Thr
—
—
Glulisine
Thr
Ilc
Asn
Lys
Pro
Glu
Thr
—
—
Detemir
Thr
Ilc
Asn
Asn
Pro
Lys‡
§
—
— ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drugs for diabetes mellitus
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access
