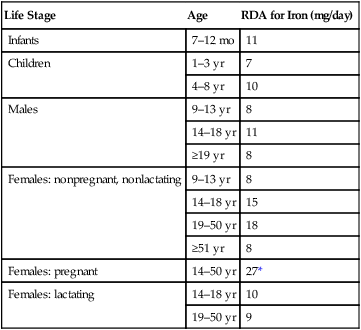
CHAPTER 55 RBCs begin developing in the bone marrow and then mature in the blood. As developing RBCs grow and divide, they evolve through four stages (Fig. 55–1). In their earliest stage, RBCs lack hemoglobin and are known as proerythroblasts. In the next stage, they gain hemoglobin and are called erythroblasts. Both the erythroblasts and the proerythroblasts reside in bone marrow. After the erythroblast stage, RBCs evolve into reticulocytes (immature erythrocytes) and enter the systemic circulation. Following the reticulocyte stage, circulating RBCs reach full maturity and are referred to as erythrocytes. The major pathways for iron movement and utilization are shown in Figure 55–2. In the discussion below, the numbers in parentheses refer to the circled numbers in the figure. As Figure 55–2 depicts, iron associated with hemoglobin undergoes continuous recycling. After hemoglobin is made in bone marrow, iron re-enters the circulation (4) as a component of hemoglobin in erythrocytes. (The iron in circulating erythrocytes accounts for about 70% of total body iron.) After 120 days of useful life, RBCs are catabolized (5). Iron released by this process re-enters the plasma bound to transferrin (6), and then the cycle begins anew. Table 55–1 summarizes the recommended dietary allowances (RDAs) of iron as a function of age. Of note, the RDA values in the table are about 10 times greater than actual physiologic need. Why? Because, on average, only 10% of dietary iron is absorbed. Hence, if physiologic requirements are to be met, the diet must contain 10 times more iron than we need. TABLE 55–1 Recommended Dietary Allowances (RDAs) for Iron *Iron requirements during pregnancy cannot be met through dietary sources alone, and hence supplements are recommended. The hallmarks of iron deficiency anemia are (1) the presence of microcytic, hypochromic erythrocytes, and (2) the absence of hemosiderin (aggregated ferritin) in bone marrow. Additional laboratory data that can help confirm a diagnosis include reduced RBC count, reduced reticulocyte hemoglobin content, reduced hemoglobin and hematocrit values, reduced serum iron content, and increased serum iron-binding capacity (IBC).* As shown in Table 55–2, iron for oral therapy is available in multiple forms. Of these, the ferrous salts (especially ferrous sulfate) and carbonyl iron are used most often. Accordingly, discussion below is limited to these iron preparations. TABLE 55–2 Iron Preparations Available for Oral Therapy Dosing with oral iron can be complicated in that oral iron salts differ with regard to percentage of elemental iron (Table 55–3). Ferrous sulfate, for example, contains 20% iron by weight. In contrast, ferrous gluconate contains only 11.6% iron by weight. Consequently, in order to provide equivalent amounts of elemental iron, we must use different doses of these iron salts. For example, if we want to provide 100 mg of elemental iron using ferrous sulfate, we need to administer a 500-mg dose. To provide this same amount of elemental iron using ferrous fumarate, the dose would be only 300 mg. In the discussion below, dosage values refer to milligrams of elemental iron, and not to milligrams of any particular iron compound needed to provide that amount of elemental iron. TABLE 55–3 Commonly Used Oral Iron Preparations
Drugs for deficiency anemias
Red blood cell development
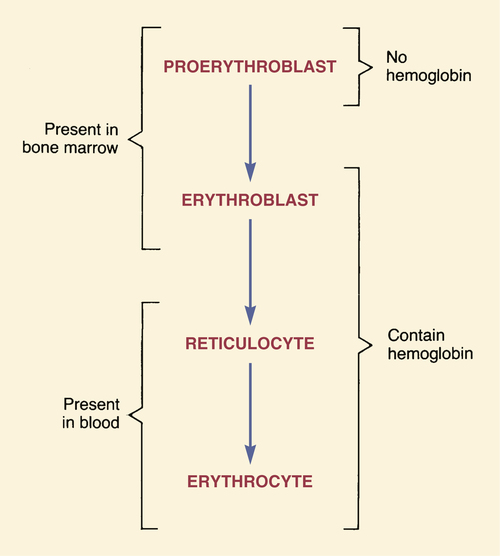
 Stages of red blood cell development.
Stages of red blood cell development.
Iron deficiency
Biochemistry and physiology of iron
Fate in the body
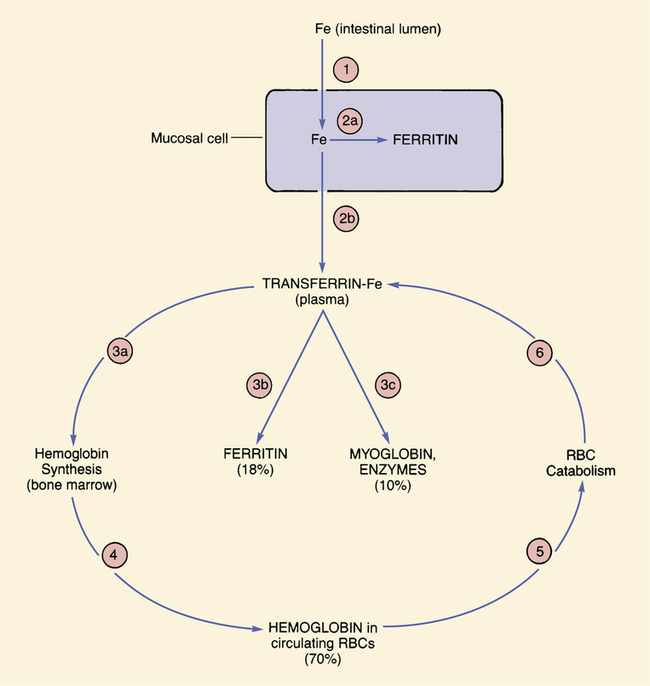
 Fate of iron in the body.
Fate of iron in the body.
Pathways labeled with circled numbers are explained in the text. Values in parentheses indicate percentage of total body stores. Elimination of iron is not shown, because most iron is rigidly conserved. (Fe = iron, RBC = red blood cell.)
Recycling.
Daily requirements

Life Stage
Age
RDA for Iron (mg/day)
Infants
7–12 mo
11
Children
1–3 yr
7
4–8 yr
10
Males
9–13 yr
8
14–18 yr
11
≥19 yr
8
Females: nonpregnant, nonlactating
9–13 yr
8
14–18 yr
15
19–50 yr
18
≥51 yr
8
Females: pregnant
14–50 yr
27*
Females: lactating
14–18 yr
10
19–50 yr
9
Iron deficiency: causes, consequences, and diagnosis
Diagnosis
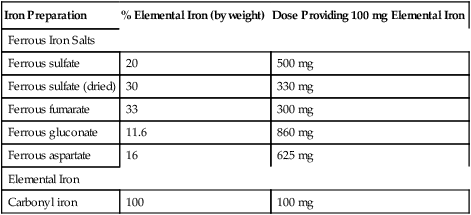
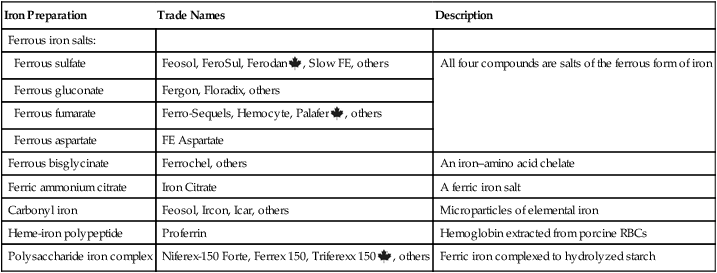
Oral iron preparations

Iron Preparation
Trade Names
Description
Ferrous iron salts:
Ferrous sulfate
Feosol, FeroSul, Ferodan ![]() , Slow FE, others
, Slow FE, others
All four compounds are salts of the ferrous form of iron
Ferrous gluconate
Fergon, Floradix, others
Ferrous fumarate
Ferro-Sequels, Hemocyte, Palafer ![]() , others
, others
Ferrous aspartate
FE Aspartate
Ferrous bisglycinate
Ferrochel, others
An iron–amino acid chelate
Ferric ammonium citrate
Iron Citrate
A ferric iron salt
Carbonyl iron
Feosol, Ircon, Icar, others
Microparticles of elemental iron
Heme-iron polypeptide
Proferrin
Hemoglobin extracted from porcine RBCs
Polysaccharide iron complex
Niferex-150 Forte, Ferrex 150, Triferexx 150 ![]() , others
, others
Ferric iron complexed to hydrolyzed starch
Ferrous iron salts
Ferrous sulfate
Dosage and administration.

Iron Preparation
% Elemental Iron (by weight)
Dose Providing 100 mg Elemental Iron
Ferrous Iron Salts
Ferrous sulfate
20
500 mg
Ferrous sulfate (dried)
30
330 mg
Ferrous fumarate
33
300 mg
Ferrous gluconate
11.6
860 mg
Ferrous aspartate
16
625 mg
Elemental Iron
Carbonyl iron
100
100 mg