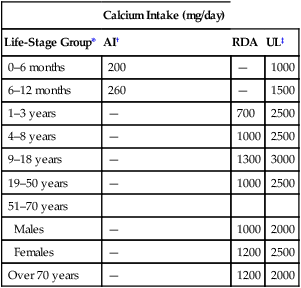
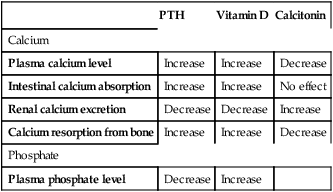
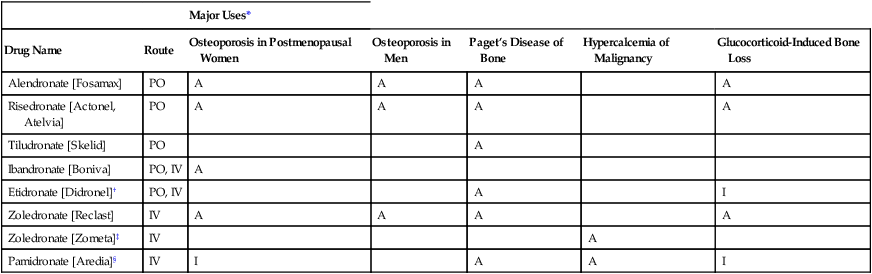
CHAPTER 75 Dairy products are good sources of calcium. For example, we can get about 300 mg from 1 cup of milk, 8 ounces of yogurt, or 1.5 ounces of cheese. Other good sources include broccoli (180 mg/cup), cooked spinach (240 mg/cup), fortified orange juice (300 mg/8 oz), and fortified cereals (250 to 1000 mg/serving). Information on the calcium content of other foods is available online at www. ucsfhealth.org/education/calcium_content_of_selected_foods/index.html. How much calcium do we need? In 2010, the Institute of Medicine (IOM) of the National Academies issued updated recommendations in a report titled Dietary Reference Intakes for Calcium and Vitamin D. As shown in Table 75–1, adolescents ages 9 through 18 need the most vitamin D: 1300 mg/day. Men and women ages 19 through 50 need 1000 mg/day. After age 50, women should increase their intake to 1200 mg/day, as should men after age 70. TABLE 75–1 Daily Calcium Intake by Life-Stage Group AI = Adequate Intake, RDA = Recommended Dietary Allowance, UL = Tolerable Upper Intake Level. *All values apply to males and females, except in the 51 to 70 age group. Calcium requirements don’t change during pregnancy or lactation. †Values for Adequate Intake are derived through experimental or observational data that show a mean calcium intake that appears to sustain a desired indicator of health, such as calcium retention in bone, for most members of the population group. AI values are employed for young children because there are insufficient data to derive an RDA. ‡The Tolerable Upper Intake Level is defined as the maximum intake that is not likely to pose a risk of adverse health effects in almost all healthy individuals in a specified group. The UL is not intended to be a recommended level of intake. There is no established benefit to consuming calcium above the RDA. Data from the Institute of Medicine of the National Academies: Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press, 2010. The vast majority of calcium in the body (more than 98%) is present in bone, in the form of hydroxyapatite crystals. It is important to appreciate that bone—and the calcium it contains—is not static. Rather, bone undergoes continuous remodeling, a process in which old bone is resorbed, after which new bone is laid down (Fig. 75–1). The cells that resorb old bone are called osteoclasts and the cells that deposit new bone are called osteoblasts. Both cell types originate in the bone marrow. In adults, about 25% of trabecular bone (the honeycomb-like material in the center of bones) is replaced each year. In contrast, only 3% of cortical bone (the dense material that surrounds trabecular bone) is replaced each year. Blood levels of calcium are tightly controlled. Three processes are involved: Regulation of these processes is under the control of three factors: parathyroid hormone, vitamin D, and calcitonin, as summarized in Table 75–2. You should note that preservation of calcium levels in blood takes priority over preservation of calcium in bone. Hence, if serum calcium is low, calcium will be resorbed from bone and transferred to the blood—even if resorption compromises the structural integrity of bone. TABLE 75–2 Effects of Parathyroid Hormone, Vitamin D, and Calcitonin on Calcium and Phosphate • Promotes calcium resorption from bone • Promotes tubular reabsorption of calcium that had been filtered by the kidney glomerulus • Promotes activation of vitamin D, and thereby promotes increased absorption of calcium from the intestine In addition to its effects on calcium, PTH reduces plasma levels of phosphate. Oral calcium preparations are used to treat mild hypocalcemia. In addition, calcium salts are taken as dietary supplements. People who may need supplementary calcium include adolescents, the elderly, and postmenopausal women. As discussed in Chapter 61 (see Box 61–1), calcium supplements may have the added benefit of reducing symptoms of premenstrual syndrome. Also, recent data indicate that calcium supplements can produce a significant, albeit modest, reduction in recurrence of colorectal adenomas. Bisphosphonates are structural analogs of pyrophosphate (Fig. 75–3), a normal constituent of bone. These drugs undergo incorporation into bone, and then inhibit bone resorption by decreasing the activity of osteoclasts. Principal indications are postmenopausal osteoporosis, osteoporosis in men, glucocorticoid-induced osteoporosis, Paget’s disease of bone, and hypercalcemia of malignancy. Bisphosphonates may also help prevent and treat bone metastases in patients with cancer (see Chapter 103). Although these drugs are generally very safe, serious adverse effects can occur, including ocular inflammation, osteonecrosis of the jaw, atypical femur fractures, and atrial fibrillation (primarily with IV zoledronate). Bisphosphonates differ with respect to indications, routes, and dosing schedules. As indicated in Table 75–5, some bisphosphonates are given PO, some are given IV, and some are given by both routes. With oral dosing, absorption from the GI tract is extremely poor. Dosing schedules vary from as often as once a day (with oral agents) to as seldom as once every 2 years (with IV zoledronate). TABLE 75–5 Bisphosphonates: Routes and Uses *A = FDA-approved indication, I = investigational use. †Also approved for prevention and treatment of heterotropic ossification. ‡Also approved for multiple myeloma and bone metastases from solid tumors.
Drugs affecting calcium levels and bone mineralization
Calcium physiology
Functions, sources, and daily requirements
Dietary sources.
Daily requirements.

Calcium Intake (mg/day)
Life-Stage Group*
AI†
RDA
UL‡
0–6 months
200
—
1000
6–12 months
260
—
1500
1–3 years
—
700
2500
4–8 years
—
1000
2500
9–18 years
—
1300
3000
19–50 years
—
1000
2500
51–70 years
Males
—
1000
2000
Females
—
1200
2500
Over 70 years
—
1200
2000
Body stores
Calcium in bone.
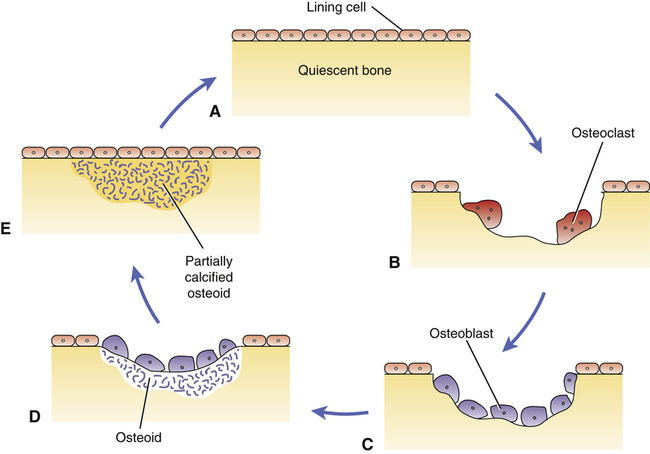
 Bone remodeling cycle.
Bone remodeling cycle.
A, Quiescent bone with lining cells covering the surface. B, Resorption of old bone by multinucleated osteoclasts. C, Osteoblasts migrate to the absorption site. D, Osteoblasts deposit osteoid, a matrix of collagen and other proteins. E, Osteoid undergoes calcification.
Physiologic regulation of calcium levels

PTH
Vitamin D
Calcitonin
Calcium
Plasma calcium level
Increase
Increase
Decrease
Intestinal calcium absorption
Increase
Increase
No effect
Renal calcium excretion
Decrease
Decrease
Increase
Calcium resorption from bone
Increase
Increase
Decrease
Phosphate
Plasma phosphate level
Decrease
Increase
Parathyroid hormone.
Drugs for disorders involving calcium
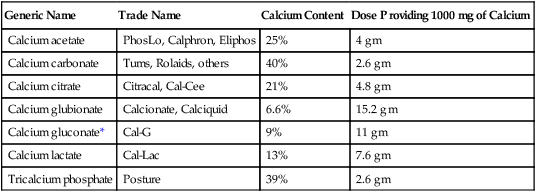
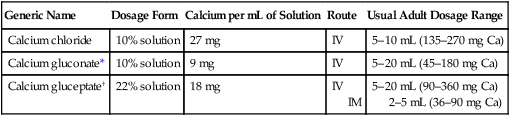
Calcium salts
Oral calcium salts
Therapeutic uses.
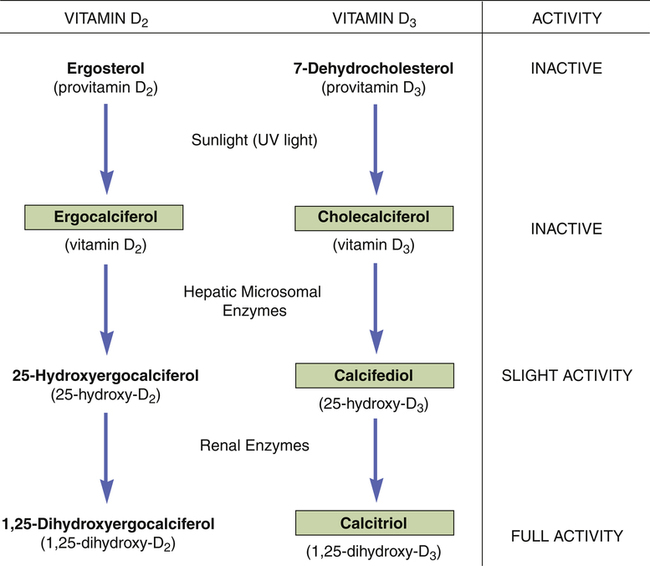
Vitamin D
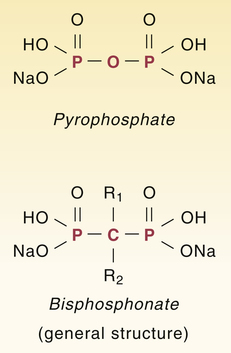
Bisphosphonates

Major Uses*
Drug Name
Route
Osteoporosis in Postmenopausal Women
Osteoporosis in Men
Paget’s Disease of Bone
Hypercalcemia of Malignancy
Glucocorticoid-Induced Bone Loss
Alendronate [Fosamax]
PO
A
A
A
A
Risedronate [Actonel, Atelvia]
PO
A
A
A
A
Tiludronate [Skelid]
PO
A
Ibandronate [Boniva]
PO, IV
A
Etidronate [Didronel]†
PO, IV
A
I
Zoledronate [Reclast]
IV
A
A
A
A
Zoledronate [Zometa]‡
IV
A
Pamidronate [Aredia]§
IV
I
A
A
I
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drugs affecting calcium levels and bone mineralization
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access