Identify the characteristics of viruses and common viral infections.
 Identify the major clinical manifestations of common viral infections.
Identify the major clinical manifestations of common viral infections.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for antiviral agents administered for herpes simplex and varicella-zoster virus.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for antiviral agents administered for herpes simplex and varicella-zoster virus.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for antiviral agents administered for cytomegalovirus.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for antiviral agents administered for cytomegalovirus.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of drugs administered for respiratory syncytial virus.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of drugs administered for respiratory syncytial virus.
 Identify the prototypes and describe their action, use, adverse effects, contraindications, and nursing implications for administration in influenza.
Identify the prototypes and describe their action, use, adverse effects, contraindications, and nursing implications for administration in influenza.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the nucleoside analog antiviral agents administered for hepatitis.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the nucleoside analog antiviral agents administered for hepatitis.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the nucleoside reverse transcriptase inhibitors administered for human immunodeficiency virus (HIV).
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the nucleoside reverse transcriptase inhibitors administered for human immunodeficiency virus (HIV).
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the nonnucleoside reverse transcriptase inhibitors administered for HIV.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the nonnucleoside reverse transcriptase inhibitors administered for HIV.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the protease inhibitors administered for HIV.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the protease inhibitors administered for HIV.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the integrase strand transfer inhibitors administered for HIV.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the integrase strand transfer inhibitors administered for HIV.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for fusion protein inhibitors administered for HIV.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for fusion protein inhibitors administered for HIV.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for CCR5 antagonists administered for HIV.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for CCR5 antagonists administered for HIV.
 Implement the nursing process in the care of the patient undergoing drug therapy for viral infections.
Implement the nursing process in the care of the patient undergoing drug therapy for viral infections.
Clinical Application Case Study
Ann Jackson calls her primary health care provider’s office. She says that she has a cold sore under her nose on the right side. Since childhood, she has had outbreaks of this infection occasionally. Her physician orders acyclovir (Zovirax) 200 mg orally every 4 hours (5 doses daily) for 5 days.
KEY TERMS
Antiretroviral drugs: antiviral medications used in the treatment of retroviral infections such as human immunodeficiency virus (HIV)
Genital herpes: a herpesvirus that appears on the genitals
Hepatitis: liver inflammation due to a virus; five different viruses cause viral hepatitis—hepatitis A, B, C, D, and G
Herpesvirus: any virus belonging to the family of the Herpesviridae
Highly active antiretroviral therapy (HAART): several combinations of antiretroviral drugs used at one time to treat HIV/AIDS
Human immunodeficiency virus (HIV): an infection caused by a retrovirus that infects the immune system, leading to acquired immunodeficiency syndrome (AIDS). There are two types of HIV virus, HIV-1 and HIV-2; most infections in the United States are caused by HIV-1 and infections with HIV-2 occur mainly in Africa
Immunocompetent: having a normal immune response
Retrovirus: virus with an RNA genome that relies on reverse transcriptase to transform its genome from RNA to DNA (e.g., HIV)
Viral load: number of HIV RNA particles in the blood; does not measure viral levels in tissues, where viral reproduction may be continuing
Introduction
Viruses cause pneumonia, hepatitis, acquired immunodeficiency syndrome (AIDS), and other disorders that affect most body systems. Many potentially pathogenic viral strains exist, including more than 150 that infect the human respiratory tract. Viral infections vary from mild, localized diseases with few symptoms to severe systemic illnesses and death. Viruses replicate in the host with the use of metabolic processes. Antiviral agents that possess a narrow spectrum of effect on the viral process have the ability to target the invading virus. This chapter describes general characteristics of viruses and viral infections. Box 21.1 provides more specific information about selected viral infections. The text then discusses antiviral agents.
BOX 21.1 Selected Viral Infections
Avian Influenza A (H5N1)
In recent years, the highly pathogenic H5N1 subtype of influenza A has been found in numerous countries. It occurs mainly in birds and poultry, but a few hundred human cases have been confirmed. Most human cases occur after exposure to infected poultry or surfaces contaminated with poultry droppings. Because human infection may cause respiratory failure and has a high mortality rate, the possibility that these viral strains might mutate so that they infect humans more easily is a major public health concern. This virus is considered the most likely cause of a future worldwide influenza epidemic.
Herpesvirus Infections
Cytomegalovirus Infection and Retinitis
Cytomegalovirus (CMV) infection is common, and most people become infected by adulthood. Infection is usually asymptomatic in healthy, immunocompetent adults. Like other herpesviruses, CMV can cause a primary infection, then remain latent in body tissues, probably for life. This means the virus can be shed in secretions of an asymptomatic host and spread to others by contact with infected saliva, blood, urine, semen, breast milk, and cervical secretions. It also means the virus may lead to an opportunistic infection when the host becomes immunosuppressed. During pregnancy, CMV is transmitted to the fetus across the placenta and may cause infection in the brain, inner ears, eyes, liver, and bone marrow. Learning disabilities and mental retardation can result from congenital CMV infection. Children spread the virus to each other in saliva or urine, whereas adolescents and adults transmit the virus mainly through sexual contact.
Major populations at risk for development of active CMV infection are patients with cancer who receive immunosuppressant drugs and organ transplant recipients, who must receive immunosuppressant drugs to prevent their body’s rejection of the transplanted organ. Patients with advanced HIV infection are also at risk, but the incidence has decreased with highly active antiretroviral therapy (HAART). Systemic CMV infection occurs mainly from reactivation of endogenous virus, although it may occur from an exogenous source. Active CMV infection may cause cellular necrosis and inflammation in various body tissues. Common manifestations of disease include pneumonitis, hepatitis, encephalitis, adrenal insufficiency, gastrointestinal (GI) inflammation, and gastric ulcerations.
In the eye, CMV infection produces retinitis, usually characterized by blurred vision and decreased visual acuity. Visual impairment is progressive and irreversible and, if untreated, may result in blindness. CMV retinitis may also indicate systemic CMV infection or may be entirely asymptomatic.
Genital Herpes Infection
Genital herpes infection is caused by the herpes simplex virus (HSV) and produces recurrent, painful, blister-like lesions on skin and mucous membranes. HSV is usually transmitted from person to person by direct contact with open lesions or secretions, including genital secretions. Primary infection occurs at a site of viral entry, where the virus infects epithelial cells, produces progeny viruses, and eventually causes cell death. After primary infection, latent virus may become dormant within sensory nerve cells. In response to various stimuli (e.g., intense sunlight, emotional stress, febrile illness, menstruation), latent virus may become reactivated and lead to viral reproduction and shedding.
In the fetus, HSV may be transmitted from an infected birth canal, and neonatal herpes is a serious complication of maternal genital herpes. Neonatal herpes usually becomes evident within the first week of life and may be manifested by the typical clusters of blister-like lesions on skin or mucous membranes. Irritability, lethargy, jaundice, altered blood clotting, respiratory distress, seizures, or coma may also occur. The lesions may heal in 1 to 2 weeks, but neonatal herpes carries a high mortality rate. In immunosuppressed patients, HSV infection may result in severe, systemic disease.
Herpes Zoster
Herpes zoster is caused by the VZV, which is highly contagious and present worldwide. Most children in the United States are infected by early school age. The virus produces chickenpox on first exposure and is spread from person to person by the respiratory route or by contact with secretions from skin lesions. Recovery from the primary infection leaves latent infection in nerve cells. Reactivation of the latent infection (usually later in life) causes herpes zoster (more commonly known as “shingles”), a localized cluster of painful and blister-like skin lesions. The skin lesions have the same appearance as those of chickenpox and genital herpes. Over several days, the vesicles become pustules, then rupture and heal. Because the virus remains in sensory nerve cells, pain can persist for months after the skin lesions heal. Most cases of herpes zoster infection occur among the elderly and the immunocompromised.
Human Immunodeficiency Virus Infection
HIV infection is caused by a retrovirus that infects the immune system. Two types of HIV virus have been identified, HIV-1 and HIV-2. Most infections in the United States are caused by HIV-1; HIV-2 infections occur mainly in Africa. HIV binds to receptors on the surface of CD4+ cells (especially T lymphocytes or helper T cells). HIV entry and replication eventually results in cell death. Because CD4+ cells play major roles in regulating immune function, their destruction results in serious impairment of the immune system.
The initial phase of HIV infection is characterized by influenza-like symptoms (e.g., fever, chills, muscle aches) that may last several weeks. During this time, the virus undergoes rapid replication. The next phase is characterized by a dramatic decline in the rate of viral replication, attributed to a partially effective immune response. During this phase, no visible manifestations of HIV infection may be present. However, replication of HIV continues and antibodies may be detected in the serum. During this period, the person is seropositive (HIV+) and infectious but asymptomatic. Eventually, the immune system is substantially damaged and the rate of viral reproduction accelerates. When viral load and immunodeficiency reach significant levels, the illness is termed acquired immunodeficiency syndrome (AIDS) and serious opportunistic infections occur. With effective drug therapy, viral load decreases and the CD4+ cell count increases, so that HIV- infected people may live for many years without progression to AIDS.
HIV infection occurs in all age groups and can spread to a new host during any phase of infection. The virus is commonly spread by sexual intercourse, by injection of intravenous drugs with contaminated needles, by mucous membrane contact with infected blood or body fluids, and perinatally from mother to fetus. Although the virus is found in most body fluids, infection has primarily been associated with exposure to blood, semen, or vaginal secretions. The virus is not spread through casual contact. Health care workers may be infected by needle-stick injuries. They should be aware that postexposure prophylaxis is available and may significantly reduce the risk of transmission.
Human Papilloma Virus (HPV) Infection
HPV infection, the most common sexually transmitted infection, is spread by contact with infected lesions. It affects both men and women and can cause genital warts and several types of cancer (e.g., cervical, vaginal, vulvar, anal, and penile). In women, 80% reportedly become infected within 5 years of becoming sexually active.
Respiratory Syncytial Virus Infection
Respiratory syncytial virus (RSV) is a highly contagious virus that is present worldwide and infects most children by school age. Epidemics of RSV infection often occur in nurseries, daycare centers, and pediatric hospital units during winter months. RSV infects and destroys respiratory epithelium in the bronchi, bronchioles, and alveoli. It is spread by respiratory droplets and secretions, direct contact with an infected person, and contact with fomites, including the hands of caregivers.
RSV is the most common cause of bronchiolitis and pneumonia in infants and causes severe illness in those younger than 6 months of age. These infants usually have wheezing, cough, respiratory distress, and fever. The infection is usually self-limited and resolves in 1 to 2 weeks. Antiviral therapy with ribavirin is used in some cases. The mortality rate from RSV infection is low in children who are generally healthy but increases substantially in those with congenital heart disease or immunosuppression. Recurrent infection occurs but is usually less severe than primary infection. In older children, RSV infection produces much milder disease but may be associated with acute exacerbations of asthma.
In adults, RSV infection causes colds and bronchitis, with symptoms of fever, cough, and nasal congestion. Infection occurs most often in those with household or other close contact with children, including pediatric health care workers. In older adults, RSV infection may cause pneumonia requiring hospitalization. In immunocompromised patients, RSV infection may cause severe and potentially fatal pneumonia.
Viral Hepatitis
There are several types of viral hepatitis, and new hepatitis viruses are still being identified. Worldwide, viral hepatitis has increased in recent years. All hepatitis viruses have similar effects on the liver, although they differ in some other characteristics. Common types of viral hepatitis are described below. Vaccines are available to prevent hepatitis A and B (see Chap. 10).
Hepatitis A virus (HAV) is transmitted mainly by the fecal-oral route and close contact with an infected person; foodborne outbreaks also occur, usually from infected food handlers or contaminated produce in the United States. The virus survives for long periods in the environment (e.g., in water and soil) and on human hands and inanimate objects. It resists freezing, detergents, and acids but can be inactivated by chlorine and temperatures above 185°F.
The HAV reproduces only in liver and GI cells, from which viral particles are released into blood and bile. The average incubation period is 25 to 30 days. The patient is infectious during the incubation period and for 7 to 10 days after symptoms develop. In infected adults, about 70% develop symptoms, including fever and jaundice, which may last as long as 2 months. Most recover without treatment and develop immunity against future HAV infections; 10% to 20% require hospitalization. In children, many are asymptomatic or develop flu-like or nonspecific symptoms without jaundice. However, even children with few or no symptoms may shed virus in their stools and be a source of community infection for as long as 6 months. The availability of hepatitis A vaccine has greatly decreased the number of reported cases. The vaccine is recommended for children and adults at increased risk of contracting the disease (e.g., travelers to certain countries, men who have sex with men, drug abusers, recipients of clotting factor replacement) and for persons with chronic liver disease.
Hepatitis B virus (HBV) can be transmitted by contact with contaminated blood and other body fluids (e.g., perinatally, during sexual contact with an infected person, by sharing needles during IV drug use, and undergoing acupuncture, hemodialysis, tattooing, or ear and body piercing). In addition, health care and public safety workers are at increased risk of developing HBV infection from injuries with contaminated equipment (e.g., contaminated needles) and from other exposures to infected body fluid. Any person exposed to infected body fluids should be evaluated for HBV infection; any person diagnosed with HBV infection should be evaluated for liver disease and for HIV infection. It is estimated that 70,000 Americans become infected with HBV every year, and approximately 5000 of them will die of the complications caused by the infection. Many Americans have chronic HBV infection; asymptomatic chronic carriers of HBV may transmit the infection to others. Hepatitis B can lead to cirrhosis, liver cancer, liver failure, and death.
The HBV can live up to 3 days on various surfaces that appear clean. The incubation period is 30 to 180 days. Increased hepatitis B surface antigens and liver enzymes (ALT, AST) occur before symptoms of HBV infection develop. After symptoms develop, antibodies to the viral antigens are produced, and the presence of antigens and viral DNA in the patient’s blood indicate that the patient is infectious.
Hepatitis C virus (HCV) can be transmitted by contact with contaminated blood or other body fluids. Persons at high risk of exposure include health care and public safety workers; IV drug users; those with multiple sex partners; those undergoing hemodialysis, tattoos, or body piercings; and those who received blood transfusions before 1990, when screening blood for HCV was started. In addition, pregnant women may transmit the virus to their infants, and mothers with symptoms and high viral titers may transmit the virus to nursing infants.
The HCV incubation period is 14 to 80 days; infection is diagnosed by testing for viral DNA or antibodies. HCV infection affects people of all ages but is most often found among 20- to 39-year-olds. Infected persons can be asymptomatic for years, but most eventually develop chronic liver disease. In the United States, HCV infection is the leading cause of cirrhosis, liver cancer, and liver transplants. Effective drug therapy may cure HCV infection.
Other hepatitis viruses (e.g., D, G, and a “transfusion- transmissible” virus) have been identified; they, like HBV and HCV, are transmitted through contact with infected blood and body fluids.
Overview of Viruses and Viral Infections
Etiology
Viruses, the infectious agents that cause viral infections, are intracellular parasites that gain entry to human host cells by binding to receptors on cell membranes. Methods of spread of viral infections include secretions from infected people, ingestion of contaminated food or water, breaks in the skin or mucous membranes, sexual contact, pregnancy, breastfeeding, and organ transplantation. All human cells do not have receptors for all viruses; cells that lack receptors for a particular virus are resistant to infection by that virus. Thus, the locations and numbers of the receptors determine which host cells can be infected by a virus. For example, the mucous membranes lining the tracheobronchial tree have receptors for the influenza A virus; helper T lymphocytes and other white blood cells have CD4 molecules, which are the receptors for the human immunodeficiency virus (HIV).
Pathophysiology
Inside host cells, viruses use cellular metabolic activities for their own survival and replication. Viral replication involves dissolution of the protein coating and exposure of the genetic material (deoxyribonucleic acid [DNA] or ribonucleic acid [RNA]). With DNA viruses, the viral DNA enters the host cell’s nucleus, where it becomes incorporated into the host cell’s chromosomal DNA. Then, host cell genes are coded to produce new viruses. In addition, the viral DNA incorporated with host DNA is transmitted to the host’s daughter cells during host cell mitosis and becomes part of the inherited genetic information of the host cell and its progeny. With RNA viruses (e.g., HIV), or retroviruses, viral RNA must be converted to DNA by an enzyme called reverse transcriptase before replication can occur. HIV is an infection caused by a retrovirus that infects the immune system, leading to AIDS.
After new viruses are formed, they are released from the infected cell either by budding and breaking off from the cell membrane (leaving the host cell intact) or by causing lysis of the cell. When the cell is destroyed, the viruses are released into the blood and surrounding tissues, from which they can transmit the viral infection to other host cells.
Viruses induce antibodies and immunity. Antibodies are proteins that defend against microbial or viral invasion. They are very specific (i.e., an antibody protects only against a specific virus or other antigen). For example, in a person who has had measles, antibody protection (immunity) develops against future infection by the measles virus, but immunity does not develop against other viral infections, such as chickenpox or hepatitis. People who are immunocompetent have intact immune systems. Most adults possess immunity to some viral diseases because they have become infected earlier in their lives. The primary infection remains latent in the tissue and is spread by blood and body fluids. Patients who are immunocompromised have impaired or weakened immune systems and may develop the infection due to decreased immunity.
The protein coat of the virus allows the immune system of the host to recognize the virus as a “foreign invader” and to produce antibodies against it. This system works well for most viruses but does not work for the influenza A virus, which can alter its protein covering so much and so often that the immune system does not recognize it as foreign to the body. Thus, last year’s antibody cannot recognize and neutralize this year’s virus.
Antibodies against infecting viruses can prevent the viruses from reaching the bloodstream or, if they are already in the bloodstream, prevent their invasion of host cells. After the virus has penetrated the cell, it is protected from antibody action, and the host depends on cell-mediated immunity (lymphocytes and macrophages) to eradicate the virus along with the cell harboring it.
Clinical Manifestations
Viral infection may occur without signs and symptoms of illness. If illness does occur, the clinical course is usually short and self-limited. Recovery occurs as the virus is eliminated from the body. Some viruses (e.g., herpesvirus) can survive in host cells for many years and cause a chronic, latent infection that periodically becomes reactivated. Also, autoimmune diseases may be caused by viral alteration of host cells so that lymphocytes recognize the host’s own tissues as being foreign.
Symptoms usually associated with acute viral infections include fever, headache, cough, malaise, muscle pain, nausea and vomiting, diarrhea, insomnia, and photophobia. White blood cell counts usually remain normal. Other signs and symptoms vary with the type of virus and body organs involved.
Drug Therapy
Scientists have developed several vaccines (see Chap. 10) to prevent viral infections as well as numerous drugs to treat HIV and other viral infections. Most of these antiviral drugs inhibit viral reproduction but do not eliminate viruses from tissues. In general, available drugs are expensive, relatively toxic, and effective in a limited number of infections. Some may be useful in treating an established infection if given promptly and in chemoprophylaxis if given before or soon after exposure. Protection conferred by chemoprophylaxis is immediate but lasts only while the drug is being taken. The remainder of this chapter describes the subgroups of antiviral drugs. Table 21.1 summarizes the medications used to treat viral infections.
Drugs for Herpesvirus Infections
DRUGS FOR HERPES SIMPLEX VIRUS AND VARICELLA-ZOSTER VIRUS
The herpesviruses include herpes simplex virus (HSV), varicella-zoster virus (VZV), and cytomegalovirus (CMV). There are two types of herpes viral infections: HSV-1 and HSV-2. HSV-1 causes fever blisters or cold sores on the lips, mouth, or face and HSV-2 causes genital warts.  Acyclovir (Zovirax), the prototype antiviral agent used to combat the herpesviruses, is an oral, parenteral, and topical antiviral drug.
Acyclovir (Zovirax), the prototype antiviral agent used to combat the herpesviruses, is an oral, parenteral, and topical antiviral drug.
Pharmacokinetics
After oral administration, the body absorbs 15% to 30% of the acyclovir dosage of acyclovir, reaching a peak of action in 1.5 to 2 hours. The drug is distributed to the tissues of the lower levels distributed to the central nervous system (CNS). It is 9% to 33% protein bound and has a half-life of 3 hours. Acyclovir crosses the placenta and enters the breast milk. Excretion of unchanged drug occurs in the urine.
Action
Following uptake by infected cells, acyclovir is converted to acyclovir monophosphate by the enzyme thymidine kinase. Acyclovir triphosphate inhibits DNA polymerase, thus interrupting viral DNA replication.
Use
Immunocompromised patients take acyclovir for initial and recurrent cutaneous and mucosal HSV and VZV. Prescribers also order the drug for the treatment of genital herpes (a herpesvirus that appears on the genitals); it decreases viral shedding as well as the duration of skin lesions and pain. Acyclovir does not eliminate inactive virus in the body and thus does not prevent recurrence of the disease unless oral drug therapy is continued. However, prolonged or repeated courses of drug therapy may result in the emergence of acyclovir-resistant viral strains, especially in immunocompromised patients. In patients with an altered immune response, authorities recommend the intravenous (IV) form for severe genital herpes, and nonimmunocompromised patients should also receive IV preparations. Table 21.2 presents route and dosage information for acyclovir and other drugs used for HSV and VZV.
 TABLE 21.2
TABLE 21.2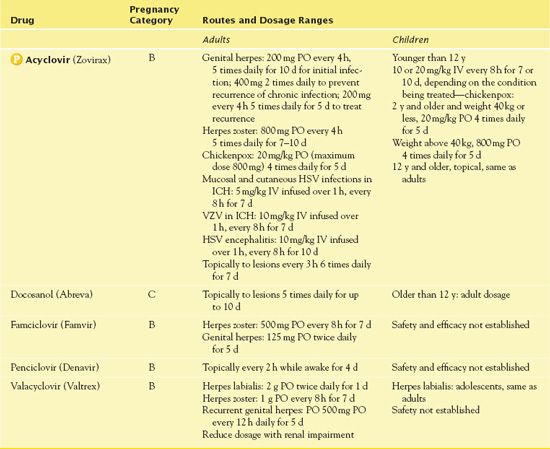
ICH, immunocompromised host.
Use in Patients With Renal Impairment
There have been reports of renal failure with acyclovir use. It is necessary to reduce the dosage of acyclovir in patients with altered renal function according to the creatinine clearance (CrCl).
Adverse Effects
Acyclovir has minimal adverse effects. The most commonly reported adverse effects are malaise, headache, nausea, vomiting, and diarrhea. The parenteral form may lead to phlebitis at the injection site, hives, itching, rash, nausea, vomiting, elevated liver enzymes, and acute renal failure. The parenteral form may result in encephalopathy, a rare but potentially serious adverse effect
Contraindications
Contraindications to acyclovir include a known hypersensitivity to the drug, heart failure, renal disease, and lactation.
Nursing Implications
Preventing Interactions
The most significant interactions with acyclovir include increased serum concentrations with probenecid, drowsiness with zidovudine, and renal insufficiency when combined with medications that cause renal toxicity.
Administering the Medication
With the topical preparation of acyclovir, it is important to wash the hands thoroughly prior to administration and apply with a gloved hand. It is crucial that the medication be prescribed and therapy started as soon as symptoms arise. With the oral medication, patients may take the drug without respect to food intake. With the parenteral form of acyclovir, it is necessary to infuse it over 1 hour to prevent renal damage. The nurse ensures that the patient is well hydrated with 2 to 3 L of fluid per 24 hours. Acyclovir is incompatible with blood products and protein-containing solutions. In patients who are obese, it is essential to calculate acyclovir dosages according to ideal body weight.
Assess for Therapeutic Effects
The nurse assesses for the healing of lesions, decreased pain, and itching. When administering acyclovir prophylactically for genital herpes, he or she assesses for fewer recurrences.
Assessing for Adverse Effects
The nurse assesses for signs and symptoms of hypersensitivity to the acyclovir. With the topical drug, he or she assesses for burning, stinging, and pruritus. With the parenteral drug, the nurse assesses for phlebitis at the injection site. It is also necessary to assess for confusion, coma, seizures, and tremors. In addition, the nurse assesses the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) for elevations as well as blood urea nitrogen (BUN) and serum creatinine for increases.
Patient Teaching
Box 21.2 identifies general patient teaching guidelines for the antiviral drugs.
BOX 21.2  Patient Teaching Guidelines for Antiviral Drugs
Patient Teaching Guidelines for Antiviral Drugs
 Prevention is better than treatment, partly because medications used to treat viral infections may cause serious adverse effects. Thus, whenever possible, it is important to use techniques to prevent viral infections, such as limiting exposure with those infected with a viral illness.
Prevention is better than treatment, partly because medications used to treat viral infections may cause serious adverse effects. Thus, whenever possible, it is important to use techniques to prevent viral infections, such as limiting exposure with those infected with a viral illness.
 Wash hands frequently and thoroughly; this helps prevent most infections.
Wash hands frequently and thoroughly; this helps prevent most infections.
 Have immunizations against viral infections as indicated.
Have immunizations against viral infections as indicated.
 With genital herpes, avoid sexual intercourse when visible lesions are present and always wash hands after touching any lesion.
With genital herpes, avoid sexual intercourse when visible lesions are present and always wash hands after touching any lesion.
 Understand that drugs may relieve symptoms but do not cure viral infections. For example, treatment of genital herpes does not prevent transmission to others, and treatment of cytomegalovirus (CMV) retinitis may not prevent disease progression.
Understand that drugs may relieve symptoms but do not cure viral infections. For example, treatment of genital herpes does not prevent transmission to others, and treatment of cytomegalovirus (CMV) retinitis may not prevent disease progression.
 Ask a health care provider for information about managing adverse drug effects.
Ask a health care provider for information about managing adverse drug effects.
 If taking foscarnet or ganciclovir for CMV retinitis, have eye examinations approximately every 6 weeks.
If taking foscarnet or ganciclovir for CMV retinitis, have eye examinations approximately every 6 weeks.
 If taking ganciclovir, maintain regular appointments for the assessment of the complete blood count and renal function.
If taking ganciclovir, maintain regular appointments for the assessment of the complete blood count and renal function.
 Administer antiviral agents for recurrent genital herpes lesions as soon as signs and symptoms begin.
Administer antiviral agents for recurrent genital herpes lesions as soon as signs and symptoms begin.
 Use gloves to apply topical antiviral ointment to lesions.
Use gloves to apply topical antiviral ointment to lesions.
Other Drugs in the Class
Docosanol (Abreva) is an over-the-counter topical antiviral agent that works in the early stages of intracellular events of viral entry into the target cells. Uses include the treatment of HSV of the face and lips. The dosage of the topical cream is five applications per day for 10 days beginning at the onset of symptoms. The patient should notify his/her primary health care provider if symptoms do not resolve in 10 days.
Famciclovir (Famvir) is an oral antiviral agent administered for herpes zoster and recurrent genital herpes. Famciclovir is metabolized to penciclovir, its active form, and excreted mainly in the urine. Drug therapy should be started within 72 hours of the appearance of a rash or within 6 hours of the onset of genital herpes lesions. A CrCl less than 60 mL/min necessitates a dosage reduction. For patients receiving hemodialysis, dosage is calculated according to CrCl, with daily doses given after dialysis. Adverse effects of the medication include purpura, headache, nausea, vomiting, dizziness, paresthesias, and constipation. Patients on extended drug therapy should have periodic complete blood counts (CBCs) to identify blood dyscrasias.
Penciclovir (Denavir) is a topical drug used for the treatment of recurrent herpes labialis. The cold sore minimally absorbs the drug. The patient should apply the ointment every 2 hours for 4 days. If the lesion does not improve, it is important to notify the primary health care provider.
Valacyclovir (Valtrex) penetrates virus-infected cells, becomes activated by an enzyme, and inhibits viral DNA reproduction. Uses include the treatment of herpes simplex and herpes zoster infections. Metabolism occurs in the liver, and excretion takes place in the kidneys. In patients with renal impairment, the drug may accumulate, produce higher blood levels, have a longer half-life, and cause toxicity.
NCLEX Success
1. A man with a fever, cough, and clear drainage from the nose presents to the clinic. A nurse practitioner diagnoses a viral infection. Which of the following describes the replication of the viral infection?
A. The RNA of an infected person has invaded the man’s mucous membranes.
B. There are breaks in the cell membrane of the infected cells.
C. Antibodies are defending against microbial cell invasion.
D. The white blood cell count increases.
2. Why would patients with genital herpes receive acyclovir (Zovirax)?
A. It decreases viral shedding and pain related to outbreak.
B. It eliminates future viral outbreaks.
C. It prevents sterility in infected patients.
D. It prevents the development of CMV.
3. A woman is using topical acyclovir (Zovirax). Which of the following is the most important intervention to instruct the patient regarding medication administration?
A. Administer the medication after meals to enhance absorption.
B. Discontinue the medication when lesions are crusted over.
C. Apply the medication to the lesions with a gloved hand.
D. Increase oral fluids to enhance healing.
4. A man has an elevated uric acid level, and he receives a prescription for probenecid. He also has cold sores on his mouth due to exposure to the sun, which is being treated with acyclovir. What effect will occur with the administration of these medications?
A. decreased serum acyclovir
B. elevated AST and ALT
C. increased serum uric acid
D. increased serum acyclovir
5. A woman experiences recurrences of herpes simplex, with an outbreak on her lips. Which medication can she apply in the early stages of the viral illness?
A. docosanol (Abreva)
B. valganciclovir (Valcyte)
C. neosporin
D. tobramycin (Tobrex)
Clinical Application 21-1
 What is a cold sore? How does acyclovir work to decrease Ms. Jackson’s symptoms and ultimately heal the cold sore?
What is a cold sore? How does acyclovir work to decrease Ms. Jackson’s symptoms and ultimately heal the cold sore?
 What patient teaching does the nurse provide to Ms. Jackson?
What patient teaching does the nurse provide to Ms. Jackson?
DRUGS FOR CYTOMEGALOVIRUS
CMV is a type of herpesvirus. The first agent developed for the treatment of CMV infection was  ganciclovir (Cytovene), the prototype. Prescribers use ganciclovir to treat CMV in immunocompromised patients, including those with AIDS and patients who have received transplants.
ganciclovir (Cytovene), the prototype. Prescribers use ganciclovir to treat CMV in immunocompromised patients, including those with AIDS and patients who have received transplants.
Pharmacokinetics
With oral administration, the onset of action of ganciclovir is 2 to 4 hours, and with IV administration, the onset of action is 1 hour. The drug is distributed widely to all tissues, including the cerebrospinal fluid (CSF) and eye tissue. The half-life is 1.7 to 5.8 hours, and in renal impairment, the half-life is prolonged. It is excreted unchanged in the urine.
Action
Like acyclovir, ganciclovir inhibits viral DNA synthesis. It is changed to a substrate that inhibits the binding of deoxyguanosine triphosphate to DNA polymerase.
Use
The IV form of ganciclovir is for the treatment of CMV retinitis in immunocompromised patients. Also, this preparation is for the treatment or prevention of CMV in transplant recipients. Patients take oral ganciclovir for the prevention of CMV if they have advanced HIV infection or are at risk for disease development. In 2009, the Centers for Disease Control and Prevention (CDC) identified an unlabeled use of intravitreal ganciclovir plus systemic foscarnet for the treatment of VZV in patients with progressive outer retinal necrosis in patients with HIV. Table 21.3 summarizes route and dosage information for ganciclovir and related drugs.
 TABLE 21.3
TABLE 21.3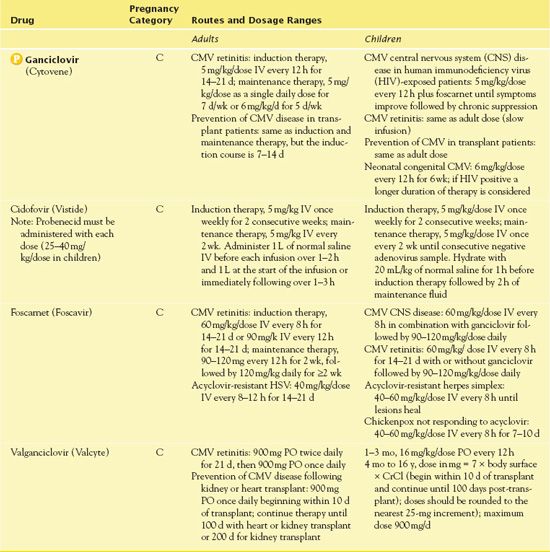
Use in Older Adult Patients
Ganciclovir administration should proceed cautiously in older adults, who often have impaired organ function and concomitant diseases and use other drugs. Renal impairment is common in older adults, and risk of toxicity is greater because excretion of the drug occurs in the kidneys. Dose reduction, when indicated by decreased CrCl, may minimize these risks.
Use in Patients With Renal Impairment
It is necessary to reduce the dosage of ganciclovir according to the CrCl. Hemodialysis patients should receive ganciclovir following dialysis. The IV dosage for dialysis patients is 1.25 mg/kg every 48 to 72 hours, with maintenance dose of 0.625 mg/kg every 48 to 72 hours.
Adverse Effects
Adverse effects of ganciclovir include chills, fever, pruritus, anorexia, nausea, vomiting, anemia, leukopenia, neutropenia, thrombocytopenia, neuropathy, retinal detachment, hematuria, and sepsis. There may be increases in BUN and serum creatinine. Ganciclovir causes granulocytopenia and thrombocytopenia in 20% to 40% of recipients, often during the first 2 weeks of therapy. The U.S. Food and Drug Administration (FDA) has issued a BLACK BOX WARNING ♦ for ganciclovir; granulocytopenia (neutropenia), anemia, and thrombocytopenia may occur. If severe bone marrow depression occurs, it is essential that the drug be discontinued; recovery usually occurs within a week of stopping the drug. A second BLACK BOX WARNING ♦ advises female and male patients of childbearing age to maintain contraceptive precautions during ganciclovir therapy and for a minimum of 90 days after drug therapy.
Contraindications
Patients should not receive ganciclovir if their neutrophil count is less than 500/mm3 or platelet count is below 25,000/mm3. A known hypersensitivity to ganciclovir or any of the antiviral agents is also a contraindication. Caution is warranted in renal impairment.
Nursing Implications
Preventing Interactions
Several drugs interact with ganciclovir. Imipenem combined with ganciclovir increases the risk of seizure activity. Amphotericin B, antineoplastic agents, didanosine, dapsone, pentamidine, probenecid, trimethoprim-sulfamethoxazole, and zidovudine administered with ganciclovir increase the risk of bone marrow suppression. Cyclosporine and ganciclovir result in nephrotoxicity. Mycophenolate, probenecid, and tenofovir increase the serum concentration of ganciclovir-valganciclovir.
Some foods and herbs interact with ganciclovir (Box 21.3).
BOX 21.3  Herb and Dietary Interactions: Ganciclovir
Herb and Dietary Interactions: Ganciclovir
 Salt/sodium
Salt/sodium
May lead to hypernatremia when combined with certain preparations of ganciclovir
 Echinacea
Echinacea
Has a potential for stimulating an autoimmune response in patients with the human immunodeficiency virus
Administering the Medication
Parenteral ganciclovir requires slow infusion, over at least 1 hour. Too rapid administration results in toxicity and excessive plasma levels. The medication is compatible with 5% dextrose and water, lactated Ringer’s, and normal saline. Administration using intramuscular, subcutaneous, or IV push is contraindicated. Ganciclovir is a hazardous medication, and it is necessary to use appropriate precautions for handling and disposal. The nurse checks the manufacturer’s guidelines and does not allow the powder or the reconstituted medication to touch the skin. The nurse does not administer the drug to patients with a platelet count less than 25,000/mm3 or a neutrophil count less than 500/mm3.
Assessing for Therapeutic Effects
The nurse assesses the patient for improvement in vision and visual acuity related to retinitis. It is also important to assess for improvement in symptoms related to pneumonia, hepatitis, encephalitis, adrenal insufficiency, and gastrointestinal (GI) inflammation or ulcerations.
Assessing for Adverse Effects
The nurse assesses BUN, creatinine, CrCl, CBC, and platelet count for impaired renal function and bone marrow depression. He or she assesses for signs and symptoms of hypersensitivity reactions. It is important to assess for neuropathic changes such as pain and diminished sensation.
Patient Teaching
Box 21.2 identifies general patient teaching guidelines for the antiviral drugs.
Other Drugs in the Class
Cidofovir (Vistide) is an IV drug indicated for treatment of CMV retinitis in patients with AIDS. After conversion to cidofovir diphosphate, it suppresses CMV replication by selective inhibition of viral DNA synthesis. Distribution in the CSF is limited. If the patient’s serum creatinine increases by 0.3 to 0.4 mg/dL, it is necessary to reduce the dose to 3 mg/kg. If the creatinine is greater than 1.5 mg/dL, it is important to discontinue therapy. The FDA has issued three BLACK BOX WARNINGS ♦ for cidofovir. The drug has possible carcinogenic and teratogenic adverse effects. It may also be nephrotoxic, and prior to administration, the patient should receive 1 L of normal saline and oral probenecid. In addition, it places the patient at risk for neutropenia.
Foscarnet (Foscavir) is an IV drug administered for CMV retinitis, acyclovir-resistant HSV, and other CMV infections related to diminished immune response. The infusion rate should not exceed 1 mg/kg/min. When given by central venous access, it is possible to administer 24 mg/mL undiluted, and in abut when given through a peripheral vein, it is necessary to dilute the solution to 12 mg/mL. The nurse gives 750 to 1000 mL of normal saline or 5% dextrose and water to initiate diuresis. The patient should be well hydrated throughout the infusion of the medication. In addition to the warning related to potential renal impairment, the FDA has issued a BLACK BOX WARNING ♦ regarding seizures with use of foscarnet, which may occur related to impaired renal function, diminished serum calcium, and CNS conditions. It is important to use foscarnet cautiously in patients with renal disease and to assess for signs of renal impairment. Manifestations of renal impairment are most likely to occur during the second week of induction therapy but may appear any time during treatment. To minimize renal impairment, it helps to monitor renal function (e.g., at baseline; two or three times weekly during induction; at least every 1 or 2 weeks during maintenance therapy) and reduce the dosage accordingly. If the CrCl drops below 0.4 mL/min/kg, it is necessary to discontinue the drug.
Valganciclovir is an oral drug administered for CMV retinitis and prevention of CMV infections following organ transplant. The medication inhibits viral reproduction after it is activated by a viral enzyme found in virus-infected cells. Valganciclovir has the same BLACK BOX WARNINGS ♦ as ganciclovir. Patients should take this drug with a high-fat diet to enhance absorption.
NCLEX Success
6. A man with HIV is on hemodialysis for renal failure. CMV retinitis develops. Which of the following is most important when administer ganciclovir?
A. Administer the medication on an empty stomach.
B. Assess the patient’s vision following the medication administration.
C. Administer ganciclovir after dialysis is completed.
D. Assess the patient’s sodium level following dialysis.
7. A patient receives a prescription for valganciclovir for the prevention of CMV infections following organ transplantation. Which of the following nursing interventions should be implemented?
A. Instruct the patient to take the medication following a meal high in fat.
B. Instruct the patient to take the meal with large amounts of fluids.
C. Instruct the patient to report abdominal pain.
D. Instruct the patient to report diminished sense of hearing.



