Injuries to the brain can be focal or diffuse. Focal injuries include contusions and hematomas. Concussions, diffuse axonal injuries, and traumatic subarachnoid hemorrhage (TSAH) are the major diffuse injuries. TBI could also be classified as penetrating or nonpenetrating. Common penetrating injuries are caused by gunshots and impalement and may result in contusions, hematoma, laceration, and cerebral edema. In all cases, the diagnosis of TBI is made with a noncontrast CT scan. In some instances, a contrast CT or an MRI may be requested after a noncontrast CT scan, but neither a contrast CT scan nor an MRI is required on an emergency basis. CT scan is more sensitive in detecting hematoma while MRI is superior in identifying DAI. In severe TBI, a follow-up CT is often ordered 3 to 5 days postinjury or as necessary to follow progress and as needed for investigation of any neurological changes.
Nearly 50% of all multi-trauma patients have a head injury. Patients with a head injury often have concurrent cervical spine, maxillofacial, or cervical vascular injuries.
Focal Cerebral Injuries: Contusions, Lacerations, and Hematomas
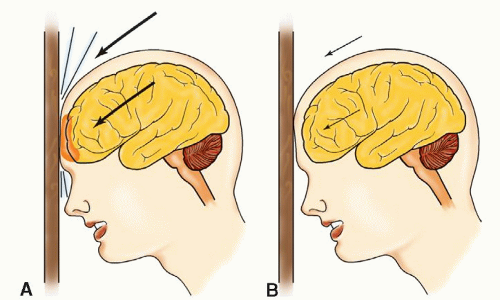
Cerebral Contusions. The anterior and middle fossae at the base of the skull have irregular, bony surfaces that are capable of contusing or lacerating the brain on impact. The distribution of
contusions in these particular areas is explained by the movement of the brain within the skull (
Fig. 16-6). The frontal and temporal poles are vulnerable because of the relative restraint created by the sphenoidal ridges and other bony irregularities of the base of the skull. Less common sites of injury are the inferolateral angles of the occipital lobes, the medial surfaces of the hemispheres, and the corpus callosum. The firm falx cerebri and tentorium cerebelli not only restrict movement of the brain, but also contribute to contusion or laceration during impact as cerebral tissue impacts on these hard surfaces. This is particularly true when the rotational force of acceleration-deceleration is applied to the frontal-temporal area. Injury to the inferolateral angles of the occipital lobe and, less commonly, the inferior surface of the cerebellar and cerebral peduncles can result from impact by the tentorium. A blow to the vertex of the head may cause cerebellar, cerebellar tonsillar, and brainstem contusions initiated by the downward thrust of the brain toward the foramen magnum.
A
cerebral contusion is a bruising of the surface of the cortex to subcortical white matter (cerebral parenchyma). Contusions may occur from blunt trauma (direct contact), a depressed skull
fracture, a penetrating wound, or an acceleration-deceleration closed injury. With acceleration-deceleration, the sites of injury are generally predictable and are located where the brain has an impact on the bony protuberances within the skull (
Fig. 16-6). These areas include the frontal poles, frontal-orbital areas, frontal-temporal junction around the Sylvian fissure (where the brain is close to the lesser sphenoidal wings), and temporal poles (the inferior and lateral surfaces of the temporal lobes where there is a shelf-like separation between the anterior and middle fossae).
The clinical effect of a contusion depends on its size and related cerebral edema. Small, unilateral, frontal lesions may be asymptomatic, whereas larger lesions may result in neurological deficits.
Contusions can cause secondary mass effect from edema resulting in increased ICP, and possible herniation syndromes. For patients with cerebral contusions, no surgical interventions are indicated and patients are managed medically with emphasis given toward prevention of secondary injuries such as ICP management.
Classification. Contusions are classified as gliding or surface contusions.
6 Gliding contusions result from rotational motion and are likely associated with DAI. Usual areas involved are the parasagittal regions of the brain with bilateral and symmetric focal hemorrhages of the cortex and surrounding white matter.
Surface contusions, on the other hand, are injuries caused by contact forces and include the following.
37
Fracture contusions occur at the sites of fractures and are particularly severe in the frontal lobes when associated with fractures in the anterior fossa.
Coup contusions are found directly under the areas of impact (contact).
Contrecoup contusions, by contrast, are cerebral injuries at the opposite pole of direct contact. A contrecoup injury is most often a contusion, but occasionally it may be a laceration. Both coup and contrecoup injuries are caused by the rapid acceleration-deceleration of the semisolid brain within the rigid cranial vault.
Herniation contusions occur at the time of injury at the point of contact where the medial part of the temporal lobe produces an impact at the edge of the tentorium or the cerebellar tonsils against the foramen magnum.
Cerebral Lacerations. A cerebral laceration refers to a traumatic tearing of the cerebral tissue. It is related to high-impact injuries and is treated in the same manner as a cerebral contusion is managed. Laceration may also be associated with a depressed skull fracture or other penetrating injuries.
Intracranial Hemorrhage/Hematomas. Traumatic intracranial hemorrhage is a common complication of TBI. Although bleeding may begin immediately after injury, its presence may not be clinically apparent until sufficient blood accumulates to cause signs and symptoms of a space-occupying lesion and mass effect. The interval between bleeding and the appearance of clinical symptoms may be minutes or weeks, depending on the site and rate of bleeding. Intracranial hemorrhage may be an occult development in a patient who has sustained a seemingly minor TBI in which consciousness has been maintained or quickly restored. Other patients with hemorrhage may be unconscious from the moment of impact.
The major types of bleeding associated with head trauma are EDH, SDH, and ICH. Other bleeding includes TSAH and intraventricular hemorrhage (IVH). Each lesion is a distinct clinical entity, but two or more types of hemorrhagic lesions can coexist.
Epidural Hematoma. EDH, also known as an extradural hematoma, refers to bleeding into the potential space between the inner table of the skull (inner periosteum) and the dura mater. It accounts for about 2.7% to 4% of traumatic head lesions. EDHs are seen most often in 20- to 30-year-old individuals or 6- to 10-year-old children. Classically, EDHs are arterial in origin, occurring from a tear of an artery in a patient who has sustained a skull fracture. The most common location for an EDH is a fracture to the thin, squamous portion of the temporal bone (90%), under which is located the middle meningeal artery. Therefore, common locations of the bleed are the temporal and temporoparietal regions, but may also occur in the frontal area. A more recent study found more EDH may be venous, the result of torn dural venous sinuses particularly in the parieto-occipital region or posterior fossa. As the bleed expands, it gradually strips away the dura from the inner table of the skull, and a large, ovoid mass develops, creating pressure on the underlying brain and causing a mass effect.
Clinically, the “classic” description of an EDH was that of a momentary unconsciousness followed by a lucid period lasting for minutes to several hours. EDH is often referred to as the “talk and die syndrome” because the patient may present with a lucid period followed by a rapid deterioration in the level of consciousness (LOC) from drowsiness to lethargy to coma as a mass effect and herniation developed. Only about 10% to 27% of patients with EDH present with the above classic manifestation. Other findings include ipsilateral pupillary dilation, contralateral hemiparesis, headache, vomiting, seizures, and hemi-hyperreflexia with a unilateral Babinski sign.
35In 18% to 44% of patients, a unilateral dilated ipsilateral pupil without loss of consciousness is found. Bradycardia and respiratory distress are late findings. If untreated, neurological deterioration may occur from drowsiness to lethargy and then to coma as mass effect and herniation developed.
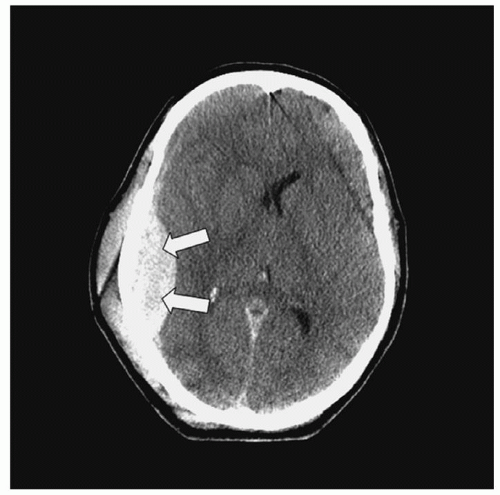
EDHs are commonly identified through CT scan of the head (without contrast), which appears as a biconvex or lenticular high-density lesion adjacent to the skull (
Fig. 16-7). Mass effect is frequently associated with this bleed, which can be seen radiographically. Lesions not noted on initial CT scan but on subsequent CT scans are known as delayed EDHs.
Immediate diagnosis and surgical evacuation of the hematoma are associated with lower mortality rate. Prognosis is associated with the severity and the duration of the brain compression caused by the EDH. Mortality is about 10%. Therefore, early diagnosis and management are important.
38 Surgical indication includes symptomatic EDH and/or acute, greater than 30 cc bleed. Presence of coagulopathy associated with a hematoma should also be identified and optimally corrected to promote hemostasis and prevent rebleeding. Hematomas of less than 30 cc with less than 15-mm thickness and a midline shift of less than 5 mm in a patient who is conscious without focal deficits can be monitored with serial CT scans and close neurologic observation.
39Subdural Hematoma. An SDH refers to bleeding between the dura mater and arachnoid or pial layer. Approximately 12% to 29% of patients with post-traumatic intracranial lesions have SDHs. Most SDHs are caused by tearing of the bridging veins located over the convexity of the brain. Other causes include tearing of small cortical arteries, cerebral contusions, and acute bleeding into chronic SDHs. Bilateral SDHs are not uncommon. SDHs are categorized based on the interval between injury and the appearance of signs and symptoms. Although there are no uniform definitions, three classes with approximate time intervals are recognized.
Acute: up to 48 to 72 hours. This consists of clotted blood that is hyperdense and crescent shaped on CT.
Subacute: 2 to 3 days to about 2 weeks. The clot now lyses, and blood products and fluid are present. Clot will be seen isodense on CT scan.
Chronic: longer than 2 weeks to several months. The clot is a fluid mass and is hypodense on CT.
Clinical presentations are usually due to brain compression and vary according to the location of the lesion and the interval between injury and presentation of symptoms. Common signs and symptoms associated with an acute SDH include gradual or rapid deterioration of the LOC from drowsiness, slow cerebration, and confusion to coma; pupillary changes; and hemiparesis or hemiplegia. Subacute SDHs are associated with less severe underlying compression. Signs and symptoms of subacute SDHs correspond closely to those of the acute SDH. Chronic SDHs can develop from seemingly minor TBIs. The time elapsed between injury and the development of symptoms may be months, so the initial injury itself may not be recalled. The lesion becomes encased within a membrane that is easily separated from the arachnoid and dura. The SDH slowly enlarges, probably because of repeated small bleeding, until a mass effect occurs. The most common symptoms of a chronic SDH include headache (progressing in severity), slow cerebration, confusion, drowsiness, and possibly seizure. Papilledema, sluggish ipsilateral pupillary response, and hemiparesis may later develop.
Elderly patients with cerebral atrophy associated with the normal aging process are prone to develop SDH because of traction on fragile bridging veins. This is particularly true with tear and rupture in the elderly with cerebral trauma. Atrophy also provides more free space into which bleeding can occur before symptoms are evident from a mass effect. With chronic SDH, the development of symptoms can be subtle because of gradual spatial compensation. Patients who have had long-term alcohol abuse with related cerebral atrophy and impaired blood clotting resulting from altered liver function and/or use of anticoagulants are also especially prone to SDH.
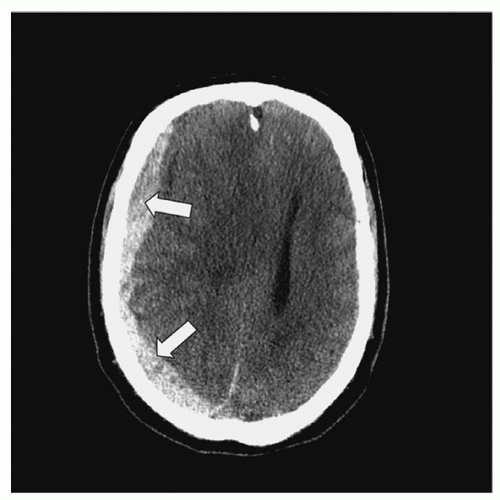
A CT scan of the head will reveal a crescentic hyperdense lesion with edema/mass effect in acute SDH. Unlike EDH, CT scan findings of acute SDH appear concave over the brain surface and more diffuse (
Fig. 16-8). The appearance of subacute and chronic SDH, as discussed earlier, varies according to density on CT scan.
Immediate surgical evacuation of the clot is recommended for symptomatic SDHs that are greater than 10 mm in thickness or greater than 5 mm midline shift. Factors that affect outcome include initial GCS score, pupillary status, time interval between trauma and treatment, clot size, mass effect, and presence of other traumatic lesions.
40 Low GCS score (<7), the presence of pupillary abnormalities, delay in treatment (>4 hours after injury), larger hematoma volume (>100 mL), midline shifts >1.5 cm, and associated cerebral contusions and/or TSAH are associated with poor functional survival and higher mortality rate. Mortality rates for patients requiring surgery are between 40% and 60%.
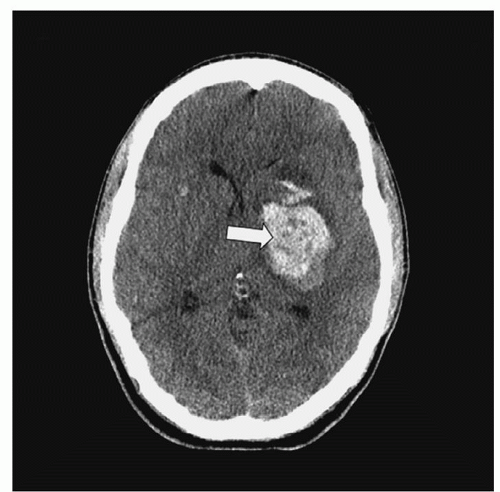
Intracerebral Hematoma. An ICH refers to bleeding into the brain parenchyma (
Fig. 16-9) resulting from contusions or blood vessel injury. Approximately 8.2% of all TBI and 13% to 35% of all severe TBI cases are due to ICH.
41 Traumatic parenchymal lesion vary with some evolving from contusions, others evolve over time (delayed traumatic ICH or DITCH); thus surgical intervention requires careful timing. Common locations are below the surface of the cortex in the white matter most commonly in the frontal and temporal lobes while others are deeper in the thalamus, basal ganglia, or parasagittal white matter. Manifestations include headache; altered mental status, which can vary from confusion to a reduced LOC or coma; contralateral hemiplegia; and ipsilateral pupil dilation.
42 Clinically, ICHs behave as expanding, space-occupying lesions and consequently cause increased ICP, which could result in herniation. ICHs are seen on CT scan as high-density lesions in the brain parenchyma.
Similarly to EDH and SDH, the size of the ICH determines the best treatment approach. Considerable controversy exists regarding the indications for surgery in ICH. This is a complex decision based on the patient’s neurological condition, size and location of the hematoma, patient’s age, and patient/family wishes. Surgery is recommended for patients with a GCS 6 to 8 with frontal or temporal contusion with a volume greater than 20 cc and with a midline shift of more than 5-mm or compressed cisterns; any lesion with a volume greater than 50 cc; or a lesion that results in refractory increased ICP. Procedures include lobectomies and decompressive craniectomies. Deeper lesions which are stable are often managed medically with supportive care and management of increased ICP.
Traumatic Subarachnoid Hemorrhage. It is estimated that between 33% and 60% of TBI patients may have a TSAH which is associated with a two-fold increased risk of death. TSAH is the accumulation of blood in the subarachnoid space and may be associated with contusions and SDH. Increased density spread thinly over the convexity causing fullness of the sulci and basal cisterns are usually noted on noncontrast CT scan of the head. This CT scan differs from the scan of aneurysmal SAH where the blood is found predominately in basal regions. The bleeding is thought to be the result of acceleration/deceleration forces that cause tearing in the pia mater on the roof of the ventricles. The distribution of the blood increases the risk of the patient developing communication hydrocephalus. Development of delayed ischemic events from vasospasm (2% to 41%) is another risk and is associated with a poor outcome. One study found that fever at admission and a contusion on CT scan were risk factors for the development of post-traumatic vasospasm.
43 Onset of symptoms varies according to location and the rate of bleeding. Clinical presentation includes headache, reduced LOC, nuchal rigidity, hemiplegia, and/or ipsilateral papillary abnormalities. Cerebral angiography is not indicated but may be done in cases when there is unclear history of trauma to exclude the presence of cerebral aneurysm.
44
Management is usually directed at supportive care and follows the treatment for aneurysmal subarachnoid hemorrhage A Cochrane review on the use of calcium channel blockers (e.g., nimodipine) has shown little to no benefit.
45 A cerebral angiogram to exclude vasospasm may be indicated in cases of delayed focal neurological deficits following TSAH.
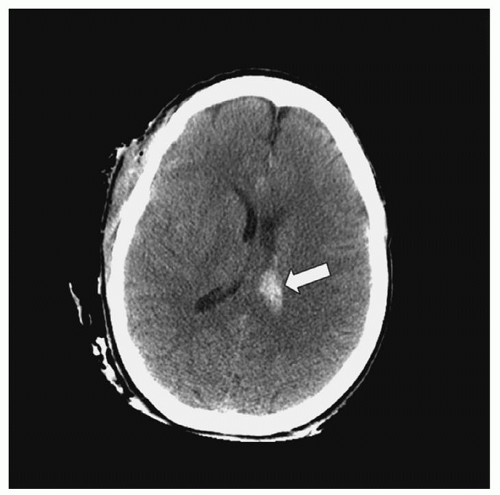
Intraventricular Hematoma. IVH (
Fig. 16-10) occurs secondary to TSAH or as an extension from an ICH and could be suggestive of severe head injury. It is reported in approximately 10% of severe head injuries.
46 Signs and symptoms include altered LOC, hemiparesis, ipsilateral pupil dilation, and intracranial hypertension.
Management consists of drainage of CSF with a ventriculostomy, ICP management, and prevention of secondary brain injuries.
Diffuse Cerebral Injuries: Diffuse Axonal Injuries
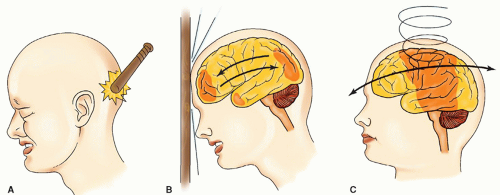
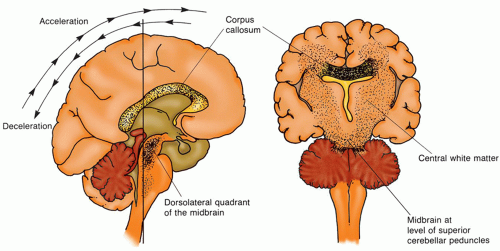
Diffuse Axonal Injury. DAI or traumatic axonal injury (TAI) is a primary TBI associated with rotational acceleration-deceleration forces during which shearing forces damage nerve fibers at the moment of injury (
Fig. 16-11). Because of the difference in acceleration gradient on certain areas of the brain during primary injury, shearing forces at the white-gray junction, corpus callosum, brainstem, and sometimes cerebellum produce diffuse tearing of axons and small blood vessels.
47,
48 Until recently, it was believed that DAI was most commonly associated with noncontact injuries. A recent study found that more patients with near-sided side impact crashes in which the head makes contact with a hard surface, sustained DAI than frontal and far-sided impact crashes.
49 DAI accounts for approximately 13.9% to 72% of primary brain injuries and 35% of deaths from all TBIs.
37,
50,
51 It is characterized by distinct gross and microscopic findings including axonal swellings that are widely distributed in the cerebral hemispheric white matter, corpus callosum, and upper brainstem; gross hemorrhagic lesions of the corpus callosum; and gross hemorrhagic lesions involving one or both dorsolateral quadrants of the rostral brainstem.
52
The angular acceleration-deceleration force produces axonal shearing (
primary axotomy) alone or together with tearing of blood vessels that cause microscopic petechial hemorrhage. In animal models, primary axotomy, in which axons are sheared, severed, or disconnected, is uncommon.
53,
54 In transected axons, axoplasmic transport from the cell body to the axon terminal continues. However, the transported material cannot pass the damaged area, where it accumulates, causing the axon to swell.
55 Most axons undergo a series of changes that result in
secondary axotomy. Although the timeline for neuronal death varies, primary axotomy usually occurs in approximately 1 hour, whereas secondary axotomy is more protracted.
Table 16-2 illustrates the proposed sequence of primary
and secondary axotomy changes due to injury.
56 Table 16-3 provides a grading system DAI that occurs with acceleration-deceleration forces to the brain. The grading system is based on the distribution of pathologic findings.
36Recent research has increased understanding of patient outcomes from DAI. Diffuse injury to axons, which are apparent microscopically throughout the white matter of the cerebral hemispheres, the cerebellum, and the brainstem, has three presentation forms related to the length of the patient’s survival.
37 In short-term survival (days), there are a large number of
axonal bulbs throughout the white matter. It takes about 15 to 18 hours for the axonal bulbs to appear in humans.
57 In intermediate survival (weeks), there are large numbers of microglia clusters throughout the white matter. In long-term survival (e.g., vegetative state), there is
long-tract degeneration of the Wallerian type in these areas. In these survivors, the degenerative effects are finally replaced by
diffuse white matter gliosis. Gliosis is the chief finding in long-term survivors and is related to severe and permanent disabilities.
58,
59A growing body of knowledge regarding location of DAI lesion on MRI is helping with prognosticating patient outcomes. The number and location of lesions help determine severity of injury and type of neurologic deficits which can be expected. Examples of these data can be found in
Table 16-4.
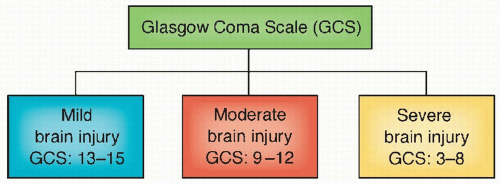
14DAI is characterized clinically by functional cerebral failure, which may range from confusion to coma and death. It is also classified
clinically as mild, moderate, or severe based on coma duration and brainstem signs.
60,
61
Mild DAI: coma lasting 6 to 24 hours with the patient beginning to follow commands by 24 hours. Outcome: death is uncommon, but cognitive and neurological deficits are common.
Moderate DAI: coma lasting longer than 24 hours, but without prominent brainstem signs. This is a common presentation (about 45% of all patients). Outcome: incomplete recovery in those who survive.
Severe DAI: coma is prolonged and associated with prominent brainstem signs (e.g., decortication, decerebration). This presentation is seen in about 36% of all DAI patients. Outcome: death or severe disability.
The clinical diagnosis of DAI is based on immediate onset of coma in a patient who has significant cerebral trauma and no intracranial lesion noted on CT scan. In many cases, an MRI may demonstrate lesions not evident on a CT. The treatment is supportive care, specifically to the unconscious patient, which will be further discussed in this chapter. DAI is associated with significant disability or death whether or not there is a coexisting traumatic lesion such as contusion or hematoma.
Concussions. Concussion or mild TBI (mTBI) is the most common TBI. A cerebral concussion is defined as an injury caused by a blunt acceleration-deceleration force (with shearing stress on the reticular formation) resulting in a brief loss of consciousness (or no loss of consciousness), and/or brief retrograde amnesia, no focal neurologic deficits, and no significant CT findings. Though the presenting symptoms may seem minor (headache, drowsiness, confusion, dizziness, irritability, giddiness, visual disturbances [seeing stars], and gait disturbances), these patients may be vulnerable to other sequelae (see Mild TBI section).
Concussions are classified as mild or classic, based on the degree of symptoms, particularly those of unconsciousness and memory loss. Mild concussion is defined as temporary neurological dysfunction without loss of consciousness or memory. By contrast, a moderate or classic concussion includes temporary neurological dysfunction, unconsciousness, and memory loss. Recovery of consciousness varies from minutes to hours. Some patients will develop a postconcussion syndrome (described later in this section).
The diagnosis of concussion is based on the patient’s history and neurological examination. CT scan or MRI is usually unremarkable, although radiologic testing is still recommended in cases where concussion is suspected to exclude any other traumatic lesion and confirm absence of any focal lesion on a CT scan, if a CT scan is done.
Depending on the severity of the concussion, a short-stay admission may be done to observe the patient’s neurological status. In other cases, a patient may be discharged home under the supervision of a responsible adult. Specific verbal and written instructions about what signs and symptoms to look for and report immediately to the physician should be provided. The patient should also be cautioned in avoiding alcohol, illicit drugs, and/or other substances such as narcotic analgesics or sedatives that may mask any signs of neurological dysfunction.
62 For any persistent and/or worsening signs and symptoms, follow-up is imperative.
Brain Injuries Caused by Gunshots and Impalement
Gunshot wounds to the head are responsible for most penetrating brain injuries, and they account for approximately 35% of deaths from brain injury in people younger than 45 years of age.
63 The wound created by the bullet depends on ballistics (i.e., the size, shape, velocity, and direction of the bullet). As the bullet propels forward and penetrates the skull, it compresses the air in front of it, thereby increasing the destruction of brain tissue locally and remotely.
64 The major effects of missile injuries are cerebral contusions and lacerations, focal tissue necrosis, hemorrhage from tearing of blood vessels, and focal or generalized cerebral edema. Hemorrhage and edema may produce increased ICP and possible herniation associated with rapid expansion during impact and in response to a space-occupying lesion. The severity of injury sustained depends on the structures involved and herniation. Cerebral abscesses are postinjury concerns because of the microorganisms that have been carried into the brain from surface debris of skin, hair, and bone fragments. Other late complications include traumatic aneurysms, seizures, and fragment migration.
Missile injuries are classified as follows.
Tangential injuries, in which the missile does not enter the cranial cavity but produces a scalp laceration, facial injury, comminuted skull fracture, meningeal tear, or cerebral contusion-laceration.
Penetrating injuries, in which the missile enters the cranial cavity but does not pass through it, resulting in the presence of metal, bone fragments, hair, and skin within the brain. There is direct injury to cerebral tissue in the path of the bullet as well as injury to tissue from the high-pressure waves created by the high-velocity bullet. This high pressure can cause coup and contrecoup injuries, spikes in ICP, and herniation.
Through-and-through injuries, in which the missile perforates the cranial contents and leaves through an exit wound. There is one tract created from a missile entering the brain, although several tracts are possible if the bullet ricochets off bony structures within the cranial vault.
The history and evidence of entrance and possible exit gunshot wounds indicate probable injury. A CT scan determines the amount of injury and identifies bone fragments and the location of bullets, if retained. Emergency surgical intervention is common for salvageable patients through evacuation of hematomas, such as EDH or SDH; to debride the wound and remove the bullet and necrotic tissue; and to treat other system injuries caused by bullets or trauma. Survivors usually require ICU management for intracranial hypertension and system complications associated with severe TBI as well of injuries to other body systems.
Penetrating injuries can also be the result of objects such as knives, sticks, scissors, nails, and the like piercing areas where the bone is thin such as the orbit. These stabbing type of injuries produce a localized laceration of the parenchyma and blood vessels rather than more extensive injury seen with gunshot wounds.