Meredith Wallace Kazer, PhD, APRN-BC On completion of this chapter, the reader will be able to: 1. Compare and contrast structural changes in the brain and nerve function associated with aging. 2. Describe functional changes in the neurologic system during the aging process. 3. Compare normal, age-related changes of the neurologic system with those associated with cognitive disorders. 4. Differentiate the symptoms of depression, delirium, dementia, and other cognitive disorders. 5. Describe the symptoms and diagnostic tests and interventions related to common neurologic disorders in older adults. 6. Use the nursing process in the development of a care plan for clients with common neurologic disorders. 7. Analyze evidence-based practice that enhances management of clients with neurologic disorders. http://evolve.elsevier.com/Meiner/gerontologic The author would also like to thank Kendra Grimes for her assistance on this chapter. The central nervous system (CNS) is divided into three major functional components: higher level brain or cerebral cortex, lower level brain (basal ganglia, thalamus, hypothalamus, brainstem, and cerebellum), and spinal cord. The brain is divided into three major areas, which include the cerebrum, brainstem, and cerebellum. The cerebrum consist of two hemispheres (right and left); each hemisphere is divided into lobes (frontal, temporal, parietal, and occipital) (Fig. 29–1). Specialized neurons located within the lobes include the hippocampus and the basal ganglia. These are the neurons that undergo structural and physiologic changes during the aging process. Another area of the CNS that undergoes significant changes in the normal aging process is the brainstem (midbrain, pons, and medulla oblongata). The reticular formation (RF) is a complex network of gray matter located primarily in the brainstem area of the CNS. The RF assists and controls many functions, including skeletal muscle movement and the sleep–wake cycle, another aspect that is altered in aging (Black, Hawks, & Hogan, 2005). The neuron is the basic unit of the CNS and functions to transmit impulses. Some neurons are motor, and some are sensory. Each neuron has a cell body (soma), dendrites, and a single axon (Fig. 29–2). Synapses are structural and functional junctions between two neurons. These are the points at which the nerve impulse is transmitted from one neuron to another or from neuron to efferent organ. The two types of synapses are electrical and chemical. Neurotransmitters are chemical substances that enhance or inhibit nerve impulses. These substances are necessary in the synaptic transmission of information from one neuron to another. In aging the function of these substances is altered because of the decrease of neurons. With aging there is also a decreased number of neurons in various areas of the brain and deposits of abnormal substances on neuronal cellular structure (dendrites) (National Institute on Aging [NIA], 2006). The loss of neurons is not as extensive in the process of aging as previously believed. In actuality, large neurons appear to shrink and few are lost. The changes in neuron function are associated with accumulation of lipofuscin (dark fluorescent pigment) granules and neuritic plaques in the cell body of some neurons and some cellular debris in neuroglia cells (Keller, 2006) (Table 29–1). TABLE 29–1 SIGNIFICANT CHANGES IN THE AGING NERVOUS SYSTEM Neuroglia cells vary in size and shape and are divided into two main classes: the microglia and macroglia (Fig. 29–3). The microglias are phagocytic scavenger cells related to macrophages that respond to infection or trauma to the CNS. The macroglia cells include astrocytes, oligodendrocytes, and ependymal cells. Astrocytes (astroglia) are star-shaped cells that provide the physical support for the neurons. They also regulate the chemical environment and nourish the neurons. These cells respond to brain trauma by forming scar tissue. CSF is a clear, colorless fluid. Approximately 135 mL of CSF circulates through the ventricles—a system of cavities within the brain—and within the subarachnoid space (80 mL in ventricles and 55 mL in the subarachnoid space). The brain and spinal cord float in the CSF, which absorbs shocks, cushions the CNS, and prevents the brain from tugging on meninges, nerve roots, and blood vessels. The choroid plexus (CP) is a group of blood vessels (capillaries) covered with a thin layer of epidermal cells. The CP is responsible for producing approximately 500 mL of CSF per day (Figs. 29–4 and 29–5). The hippocampus is a part of the temporal lobe that plays an important role in memory and learning. Normal aging is associated with changes in the ability to consciously learn and retain new information easily. This occurs as a result of structural changes, synapse loss in the neurons, decreased microvascular integrity, reduction in glucose metabolism, and alterations in the neuroglia cells with aging. As a result of changes in the secretory pattern of the hypothalamic–pituitary–adrenal (HPA) axis, additional alterations occur in the hippocampal area of the brain. The hippocampal area is strongly influenced by HPA hormones. The specific aspects altered by the aging process are the explicit memory (e.g., delayed recall), the ability to learn new information quickly, memory storage, and memory retrieval (Keller, 2006). A reduction in the turnover of CSF with age decreases the distribution and efficiency by which the necessary substances are delivered from the CP to the brain target sites. These substances include the hormones necessary for metabolism and appetite and the nutrients (e.g., transferrin, glucose, amino acids, and vitamins) necessary for nerve function. A reduction in the turnover of CSF can affect the removal of waste products, toxins (e.g., amyloid peptides and lactate), and drugs. The accumulation of these substances resulting from age-related changes may contribute to diseases causing cognitive decline. One significant factor that reduces the turnover secretion rate of CSF is the age-related increase in resistance from the vascular (sagittal venous sinus) system in the arachnoid (Redzic, Preston, Duncan, et al 2005). These changes occur in various degrees among aging individuals. The age-related neurodegenerative and neurochemical changes in the cerebellum are believed to be the underlying cause of decline in motor and cognitive function. The neurodegenerative and neurochemical changes, combined with inner ear and vestibular changes, cause many elderly to experience changes in their balance. These changes can further contribute to postural hypotension because of an inability to quickly respond to changes in position. The symptoms of postural hypotension are dizziness or lightheadedness when changing positions rapidly. However, compensatory processes in the cortex and subcortical areas of the brain help aging individuals maintain relatively normal motor performance (Heuninckx, Wenderoth, & Swinnen, 2008). Normal sleep is organized into different stages that cycle throughout the night. The sleep stages are classified into the following categories (Brannon, 2008): • Rapid eye movement (REM) sleep. This is the stage of sleep during which muscle tone decreases significantly. In advanced aging REM sleep is maintained without much decline. • Non-REM sleep. This is subdivided into four stages. Stages 1 and 2 constitute light sleep, and stages 3 and 4 are deep sleep or slow-wave sleep. In aging there is an increase in the duration of stage 1 sleep and an increase in the number of shifts into stage 1 sleep. Stages 3 and 4 decrease significantly with age. Among the oldest old people (older than 90 years), stages 3 and 4 may disappear completely. Some older women have normal or even increased stage 3 sleep, whereas men have normal or reduced stage 3 sleep. Excessive daytime somnolence is not part of normal aging. Somnolence indicates the presence of a pathologic condition. In the aging process there is a decrease in hypothalamic function; as a result, older clients have been observed to be more easily aroused from sleep by auditory stimuli, which suggests increased sensitivity to environmental stimuli and altered neuroendocrine function (Brannon, 2008). The somatic receptors respond to touch, pressure, cold, pain, and body position. These receptors also become less sensitive as aging occurs. Older individuals therefore experience a decreased ability to feel pain and cope with temperature changes. These and additional age-related changes are presented in Table 29–1. The assessment of neurocognitive function is an essential part of a comprehensive assessment in older adults. Neurocognitive function assessment includes several components and can be easily incorporated into the general assessment of older adults through history taking, physical examination, and the use of selected screening instruments. A complete mental status assessment should include attention, memory, orientation, perceptions, thought processes, thought content, insight, judgment, affect, mood, language, and higher cognitive functions (see Chapter 14 for the components of a cognitive or mental status assessment). Neurologic assessment includes the evaluation of cranial nerves, gait, balance, distal deep tendon reflexes, plantar responses, primary sensory modalities in the lower extremities, and cerebrovascular integrity. Complete neurocognitive examinations should be performed on all older adults to establish baseline function and to detect potentially reversible conditions causing mental and behavioral disturbances. Few older adults recognize the symptoms of cognitive decline in themselves. It is often a friend or family member who reports these symptoms to the nurse or physician caring for the client. An interview with the friend or family member, physical assessment, and the use of structured mental status assessments assist the nurse in identifying cognitive decline in older adults (Elliott, Horgas, & Marsiske, 2008). One screening tool that has been used to identify the presence and severity of dementia symptoms based on level of function and cognition in older adults is the Dementia Severity Rating Scale (DSRS) (Harvey, 2005). The DSRS is an 11-item instrument that can be easily and quickly administered and covers the areas of memory, orientation, judgment, community affairs, home activities, personal care, speech and language recognition, feeding, incontinence, and mobility or walking. A normal score on this instrument is four or less; the score increases as the older person’s cognition decreases. The MiniMental State Examination (MMSE) is one of the most widely used instruments in all settings and is useful for the assessment of orientation, immediate and recent memory, attention, calculation, and language and motor skills (Folstein, Folstein, & McHugh, 1975). Cognitive impairment is identified in individuals with scores of less than 24; however, educational level and age may have an impact on the results and must be taken into consideration when interpreting the results. The Mental State Questionnaire (MSQ) is a 10-item list, one of the shortest instruments used to assess cognitive function. This test is reliable in identifying clients with moderate to severe cognitive impairment. The MSQ is not beneficial for all client evaluations (Alexopoulos & Mattis, 1991). The Blessed Dementia Scale or Short Blessed Test (SBT) is another frequently used screening tool for the assessment of dementia; however, both aging and depression have an effect on Blessed Orientation-Memory-Concentration (BOMC) test performance (Jorm & Jacob, 1989). The BOMC test is a reduced version of the SBT and consists of six questions. This shortened version of the SBT is used by many disciplines. The Geriatric Depression Scale is widely used to identify depression in older adults (Yesavage et al, 1983). Patients answer 30 statements on this screening tool with either “yes” or “no.” A score of 11 or greater indicates possible depression and the need for more in-depth evaluation. See Chapter 14 for an in-depth discussion on the evaluation of depression in older adults. The rate of depression among older adults has remained relatively stable over the past decade with approximately 12% of individuals older than the age of 65 reporting depressive symptoms (Federal Interagency Forum on Aging-Related Statistics, 2008). However, as one ages the rate of depression increases. The percentage of men older than the age of 85 reporting depressive symptoms is almost double that of men aged 65 to 74 (Federal Interagency Forum on Aging-Related Statistics, 2008). In older age, depression is associated with higher suicide rates than in the younger depressed population. Although older Americans make up 13% of the U.S. population, they account for 16% of all suicide deaths (National Institute of Mental Health, 2009). White men older than 50 years of age have the highest rate of suicide at approximately 50 suicide deaths per 100,000 men (National Institute of Mental Health, 2009). Dombrovski and Szanto (2005) report that older adults in the United States, especially the depressed elderly, are more likely to commit suicide than any other age group, although it is difficult to estimate the true incidence of suicide. Depression may manifest itself through signs such as fatigue; constipation; psychomotor retardation; depressed mood; loss of interest, energy, libido, or pleasure; changes in appetite, weight, and sleep patterns; and agitation; anxiety; or crying (American Psychiatric Association [APA], 2004). Depression often is first seen in older adults as cognitive impairment, particularly in the areas of attention and concentration. Depressed older adults may neglect eating or caring for a chronic medical condition, predisposing them to the development of delirium. Depression is also a common response to serious illness of any kind, particularly multiple sclerosis, hypothyroidism, lupus, hepatitis, acquired immunodeficiency syndrome (AIDS), vitamin deficiencies, and anemia. These conditions may produce depression in a more direct biologic sense. Drugs can also contribute to depressive symptoms (Box 29-1). Older adults require a careful medical history and physical examination before the diagnosis of depression can be made. Late-life depression is often similar in presentation or may be concomitant to cognitive impairment and dementia caused by neurochemical changes and awareness of the loss of physical or intellectual functioning. Symptoms common to both depression and dementia include irritability, inability to concentrate or feel pleasure, loss of interest in life, and lack of energy and initiative. The term pseudodementia has been used to describe depression masquerading as dementia. Pseudodelirium is the term used when an older adult is seen with an acute confusion found to be due to depression. With a careful assessment, it is possible to make the appropriate diagnosis. Individuals with dementia are more likely to show signs of disorientation and loss of short-term memory and are less likely to feel sadness or guilt or to complain about pain, insomnia, and poor appetite. Table 29–2 offers a comparison of selective features associated with dementia, delirium, and depression. Refer to Chapter 14 for a comprehensive discussion of depression among older persons. TABLE 29–2 CLINICAL FEATURES OF DEPRESSION, DELIRIUM, AND DEMENTIA Delirium is described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (APA, 2000) as a transient, organic mental syndrome characterized by a reduced level of consciousness, reduced ability to maintain attention, perceptual disturbances, and memory impairment. The onset of delirium is short, generally ranging from hours to days. Delirium occurs in all settings, including homes, assisted living facilities, nursing facilities, and hospitals. Frequently, when an older adult becomes delirious in a community setting, it precipitates a hospital admission, in part because of the underlying illness causing the delirium. Delirium occurs in 7% to 61% of older hospitalized clients, with associated morbidity rates ranging from 6% to 18% within this group (Royal College of Psychiatrists, 2005). The risk factors for delirium include advanced age, CNS diseases, infection, polypharmacy, hypoalbuminemia, electrolyte imbalances, trauma history, gastrointestinal or genitourinary disorders, cardiopulmonary disorders, and sensory changes. These factors can lead to physiologic imbalances increasing the risk for confusion (Fick & Mion, 2008). Specific laboratory testing should be guided by clues in the history and physical examination so that the physiologic causes of delirium can be identified. Early delirium research focused on the timely identification of delirium in hospitalized older adults. Current research focuses on the identification of risk factors and prevention strategies. Multiple instruments have been developed to assess for delirium. The acute confusion assessment instruments for nursing include the Clinical Assessment of Confusion-A&B (Vermeersch, 1986; 1992), the VAS-AC (Nagley, 1986), and the NEECHAM Confusion Scale (Neelon et al, 1996). There are also several medical-model delirium assessment scales, including the Delirium Rating Scale (Trzepacz, Baker, & Greenhouse, 1988), Delirium Symptom Interview (Levkoff, Besdine, & Wetle, 1986), and Confusion Assessment Method (CAM) (Inouye et al, 1990). All these instruments have demonstrated sensitivity and specificity for use with hospitalized older adults. In one study, a program was developed for the early detection and treatment of older persons who developed symptoms of delirium during hospitalization (Inouye et al, 2007). Among the study participants older than the age of 70, 11.8% had delirium on discharge as measured by the CAM. The predictive model used in this study found five risk factors for delirium: cognitive impairment, visual impairment, functional impairment, comorbidity, and the use of physical restraints. The study validated the previously studied predictive model, and the authors concluded that at least four of the five risk factors for delirium are amenable to intervention. Table 29–3 outlines the assessment and intervention protocols used in the care of patients with delirium. TABLE 29–3 RISK FACTORS FOR DELIRIUM AND INTERVENTION PROTOCOLS Nonpharmacologic sleep protocol: at bedtime, warm drink (milk or herbal tea), relaxation tapes or music, and back massage Sleep-enhancement protocol: unit-wide noise-reduction strategies (e.g., silent pill crushers, vibrating beepers, and quiet hallways) and schedule adjustments to allow sleep (e.g., rescheduling of medications and procedures) ∗Orientation score consisted of results on first 10 items on the MiniMental State Examination (MMSE). †Sedative drugs included standard hypnotic agents, benzodiazepines, and antihistamines, used as needed for sleep. From Inouye SK: Risk factors for delirium and intervention protocols, N Engl J Med 340(9):669, 1999. There are a number of interventions to prevent delirium in hospitalized clients. Assessment with the use of a validated instrument such as the CAM is the first line in preventing and treating delirium. Modifying risk and maintaining safety are key features of delirium care (Fick & Mion, 2008). An early study by Inouye (1999) resulted in the development of a broad spectrum of preventive interventions that may be modestly effective, including psychiatric or medical assessment, support, education, and reorientation. Inouye also found that interventions by nurses alone were as effective as interventions by physicians. Delirium management includes rapid diagnosis and treatment of the underlying cause, management of disruptive behaviors, and supportive care. As discussed in the Inouye et al study (2007), assessment of changes in older persons’ cognition is paramount. A thorough history and physical examination are essential for the identification of the onset, cause, direct physiologic manifestation of a general medical condition, or intoxication with or withdrawal from substances that may be contributing to the onset of delirium (American Psychiatric association, 2004). The treatment of delirium entails the identification and treatment of the underlying cause. These treatment interventions may be categorized as pharmacologic and nonpharmacologic. Nonpharmacologic approaches include promoting activity, improving nutritional and fluid intake, decreasing sensory overstimulation or deprivation, and reassuring the older adult and his or her family members (Fick & Mion, 2008). Pharmacologic approaches may include antibiotics to treat underlying infections and the removal of potentially contributory medications. It may be necessary to use medications for the management of agitation and hallucinations (haloperidol) or alcohol withdrawal symptoms (benzodiazepines). The former can be used judiciously in the treatment of agitation and hallucinations, but polypharmacy should be avoided (see Evidence-Based Practice Box). In 2002, roughly 2.5 million Americans were diagnosed with dementia. By 2030, Thurman reports in the State of Aging and Health in America that the number of Americans diagnosed with dementia will more than double to 5.2 million (Centers for Disease Control and Prevention [CDC], 2007). Not included in these statistics is the phenomenon of potentially reversible dementia. The primary types of dementia include Alzheimer’s disease (AD), vascular dementia (VaD), dementia with Lewy bodies (DLB), and frontotemporal dementia (FTD). Dementia is a syndrome of gradual and progressive cognitive decline. It has been defined as an alteration in memory in addition to acquired persistent alteration in intellectual function (e.g., orientation, calculation, attention, and motor skills) compromising multiple cognitive domains. In dementia, individuals are unable to do the things they used to do because of the mental changes associated with this disease process. Dementia may involve language deficits, apraxia (difficulty with the manipulation of objects), agnosia (inability to recognize familiar objects), agraphia (difficulty drawing objects), and impaired executive function (Alzheimer’s Association, 2007). Reversible dementia is a phenomenon that occurs when other pathologic conditions masquerade as dementia. Causes of potentially reversible dementia are presented in Box 29-2. It is important to identify and treat the underlying causes of dementia symptoms, but even if these disorders are identified and treated, not all individuals with dementia symptoms will improve (Koedam, 2008). AD is the most common form of dementia in older persons and accounts for 60% to 80% of individuals participating in research on dementia (Alzheimer’s Association, 2007). AD is a progressive, neurodegenerative disease characterized by the presence of neurofibrillary tangles composed of misplaced proteins within the brain, cortical amyloid plaques, and granulovascular degeneration of neurons in the pyramidal cell layer of the hippocampus. An estimated 5 million Americans have AD, and it is predicted that by 2050 the number of individuals with AD could rise to 13.4 million (Alzheimer’s Association, 2006). AD is the seventh leading cause of death in the United States (CDC, 2007). The personal and public price of AD is high. Medicare costs for beneficiaries with AD are expected to exceed the ability to absorb the cost (Alzheimer’s Association, 2007). One risk factor for the development of AD is genetics, particularly in cases of presenile dementia, or dementia seen in individuals younger than the age of 65. Other genetic mutations causing excessive accumulations of amyloid protein are also associated with age-related (sporadic) AD (Waring & Rosenberg, 2008). AD may show autosomal dominant inheritance in families with presenile dementia. Chromosome 14 has been linked to early-onset familial AD in more than 70% of cases, and chromosome 19, closely associated with the gene responsible for the encoding of apolipoprotein E, has been linked to late-onset familial AD (Waring & Rosenberg, 2008). Mutations in chromosome 21, the gene associated with Down syndrome (trisomy 21), have also been linked with development of the disease. All persons with Down syndrome who survive to the third or fourth decade develop the pathologic condition of AD (Jones et al, 2008). In spite of these data, AD is not an exclusively inherited disease. Environmental factors that are potentially associated with cognitive dysfunction include exposure to toxic substances or chemicals. The role of environmental factors in the expression of AD is supported not only by studies that reveal only a 40% concordance rate for AD in monozygotic twins but also by variations in age at the onset of dementia in monozygotic twin pairs. These findings suggest that nongenetic and potentially environmental factors may influence the development of AD. Head trauma occurring early in life with associated loss of consciousness has been associated with the development of AD in later years (Van Den Heuvel, Thornton, & Vink, 2007). Reports suggest an association between AD and vitamin B12 deficiency because low serum vitamin B12 concentrations are found in more than 10% of older people and the prevalence of low serum vitamin B12 levels has been reported among people with AD. Hyperhomocysteinemia (plasma homocysteine of greater than 14 μmol/L) increases the risks for dementia and AD (Smith, 2008). Currently no validated test is available for the diagnosis of AD. Although autopsy remains the gold standard for the diagnosis of AD, clinical diagnosis has become increasingly accurate over the past several years (Alzheimer’s Association, 2007). Magnetic resonance imaging (MRI) and positron emission tomography (PET) scans have been used to identify the hippocampal atrophy associated with the diagnosis AD. Because the costs are prohibitive and the findings are similar to those of clinical examination for the diagnosis of AD, these tests are not routinely recommended (Alzheimer’s Association, 2007). At this time there is no cure for AD. Several pharmacologic options have been introduced to slow the progression of the disease in its early stages. These new medications have transformed the care of AD clients. Tacrine (Cognex) was the first of the cholinesterase inhibitors, but because of the need to frequently monitor a client’s liver function, its use is limited. Donepezil (Aricept), rivastigmine (Exelon), and galantamine (Reminyl), all cholinesterase inhibitors, were originally found to have fewer side effects and demonstrated greater cognitive and global functional improvement in early and midstage AD. These medications may keep some symptoms from becoming worse for a limited time. However, recently a study of 19,803 community-dwelling older adults with dementia receiving cholinesterase inhibitors had more frequent hospital visits for syncope and syncope-related events compared with 61,499 healthy control subjects (Gill et al, 2009). A fifth drug, memantine (Namenda), was approved for use in the United States. Combining memantine with other AD drugs promises to be more effective than any single therapy. Although cholinesterase inhibitors have been useful for older adults with AD, they have not been shown to have the same effects in older adults with other types of progressive dementia. An herbal plant extract from Ginkgo biloba has shown promise in stabilizing and occasionally improving cognitive performance and function in demented older adults for 6 months to a year (Birks & Grimley Evans, 2009). It has been used as a standardized form in Europe for many years but is sold in the United States as a nutritional supplement. Vitamin supplementation has been attempted and studied, but there is no consensus on the efficacy of this intervention. In a research review by Jia, McNeill, and Avenell (2008), there was insufficient evidence to support the efficacy of vitamin B12 in improving the cognitive function of people with dementia and low serum B12 levels. In another review, Malouf and Grimley Evans (2008) found no benefit from folic acid with or without vitamin B12 in comparison with placebo on any measures of cognition or mood in the clinical trials they reviewed. The results of a 2004 metaanalysis of the role of vitamin E in the treatment of AD were inconclusive (Isaac, Quinn, & Tabet, 2008). Although nonsteroidal antiinflammatory medications (NSAIDs) have been suggested in the treatment of AD, no evidence exists from randomized double-blind and placebo-controlled trials demonstrating their benefit (Vlad, Miller, Kowall, & Felson, 2008). Because ibuprofen and other NSAIDs have a significant side effect profile, including gastrointestinal bleeding, it needs to be demonstrated that the benefits of such a treatment outweigh the risk of side effects before ibuprofen can be recommended for AD treatment. VaD is the second most frequently occurring type of dementia among older persons (Schneck, 2008). Often referred to as multiinfarct dementia, VaD is defined as a loss of cognitive function resulting from ischemic, hypoperfusive, or hemorrhagic brain lesions resulting from cerebrovascular disease or cardiovascular pathologic conditions. VaD is associated with the progressive loss of brain tissue as a result of a series of small brain attacks (infarcts) caused by occlusions and blockages within the arteries to the brain. Individuals who have experienced a cerebrovascular accident (CVA) have an even greater risk of VaD. Research on the use of donepezil for improving cognitive function, clinical global impression, and ability to perform ADLs in patients with mild to moderate VaD has been promising (Dichgans et al, 2008). Nimodipine (Nimotop), a calcium channel blocker, has also demonstrated short-term benefit in the treatment of VaD; however, little evidence supports its efficacy with long-term use in VaD (Pantoni et al, 2005). There is insufficient evidence to support interventions for the tertiary prevention of VaD. Zekry (2009) supports the need for well-designed, rigorous clinical trials with better defined assessment criteria for VaD. DLB is a progressive, degenerative brain disorder named after an intracytoplasmic neuronal inclusion, which may be found in the brainstem, diencephalon, basal ganglia, and cerebral cortex (Kalra, Bergeron, & Lang, 1996). DLB is estimated to account for up to 30% of all dementia cases (Zaccai, McCracken, & Brayne, 2005). Individuals with Parkinson’s disease (PD) have a sixfold increased risk for the development of DLB compared with the general population (Buter et al, 2008). Management of patients with DLB focuses on symptomatic relief when psychiatric and behavioral symptoms become distressing. Treatment for PD is essential in the event of gait and balance alterations. The use of cholinesterase inhibitors has also been supported in DLB (Bhasin, Rowan, Edwards, & McKeith, 2007). It is important to note that recent case reports reveal a possible exacerbation of DLB related to administration of memantine (Ridha, Josephs, & Rossor, 2005). Thus, careful diagnosis and care management is essential for preventing this drug–disease interaction. Caregiver education and support are important aspects of disease management because of the unique pattern of psychiatric symptoms and motor and cognitive deficits that these patients display. FTD is a clinical syndrome of exclusion associated with non-AD pathologic conditions and is relatively rare in the clinical setting. This syndrome includes the spectrum of non-AD dementias and is characterized by focal atrophy of the frontal and anterior temporal regions. Pathologically, FTD is variable; some cases may show tau-positive disease (with or without classic Pick bodies), whereas others show ubiquitin-positive inclusions, and still others may lack distinctive histologic features (Mendez et al, 2008). Two major clinical presentations of FTD include frontal or aphasic variants. Frontal behavioral variant FTD is associated with progressive changes in personality and social cognition, disinhibition, loss of empathy, changes in eating patterns, ritualized or stereotypic behaviors, and apathy. Aphasic variants of FTD include progressive fluent or nonfluent aphasia (loss of ability to use language), depending on the frontal or temporal focus (Mendez et al, 2008). Either of these main variants may be associated with motor neuron disease, although the behavioral features typically precede motor symptoms (Mendez et al, 2008). Normal pressure hydrocephalus (NPH) is a rare but potentially reversible condition; if left untreated, it leads to permanent cognitive impairment. In NPH the CSF circulates to the cerebral subarachnoid space, enlarging the ventricles but causing no rise in the CSF pressure. It is believed that the majority of cases of NPH are related to prior cerebral insults such as traumatic injury, viral insult, or previous surgery. NPH has a triad of symptoms that present together: gait disturbance (e.g., ataxic or magnetic gait), urinary incontinence, and cognitive dysfunction (Shprecher, Schwalb, & Kurlan, 2008). Clients who develop dementia before the gait disturbance have poorer outcomes. Treatment involves placing a shunt to drain the CSF (Shprecher, Schwalb, & Kurlan, 2008).
Cognitive and Neurologic Function
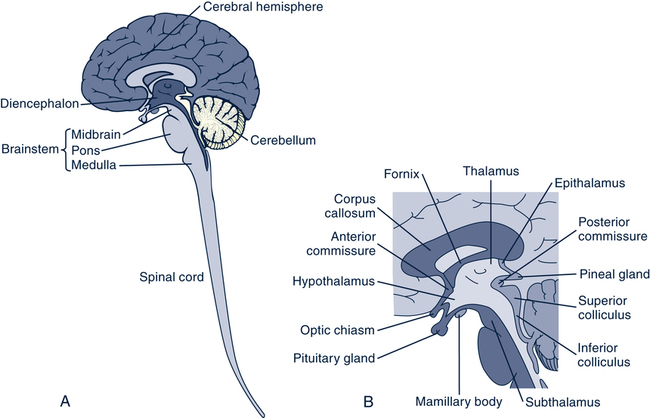
Structural Age-Related Changes of the Neurologic System
Cellular and Structural Changes
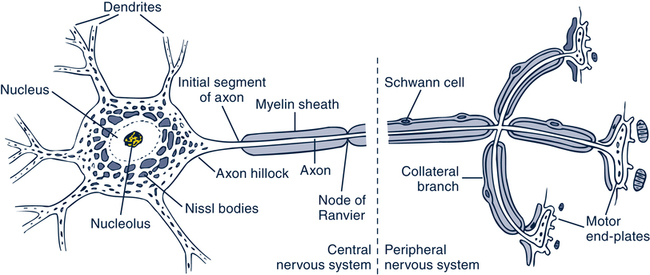
Neuron
Neurotransmitters
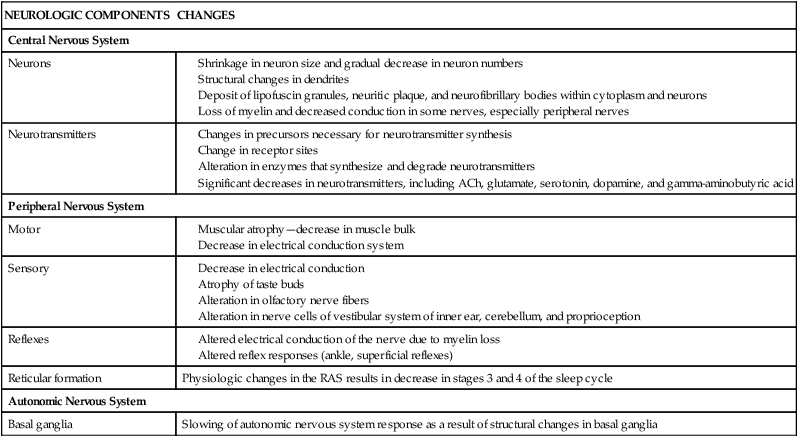
NEUROLOGIC COMPONENTS
CHANGES
Central Nervous System
Neurons
Neurotransmitters
Peripheral Nervous System
Motor
Sensory
Reflexes
Reticular formation
Physiologic changes in the RAS results in decrease in stages 3 and 4 of the sleep cycle
Autonomic Nervous System
Basal ganglia
Slowing of autonomic nervous system response as a result of structural changes in basal ganglia
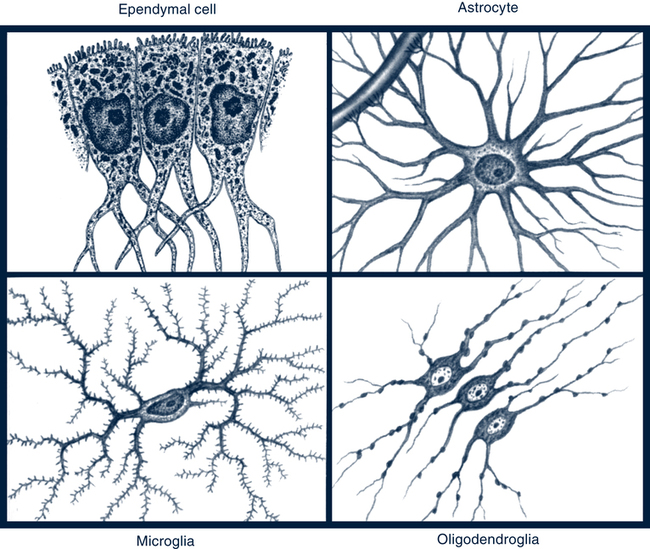
Neuroglia and Schwann Cells
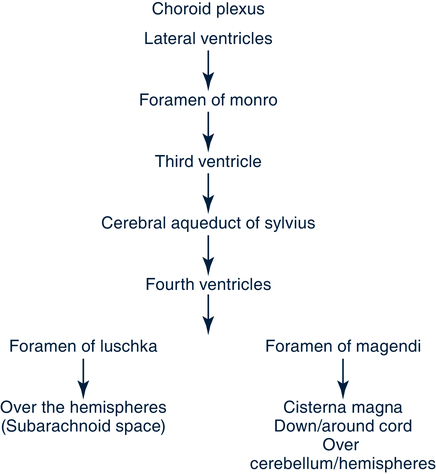
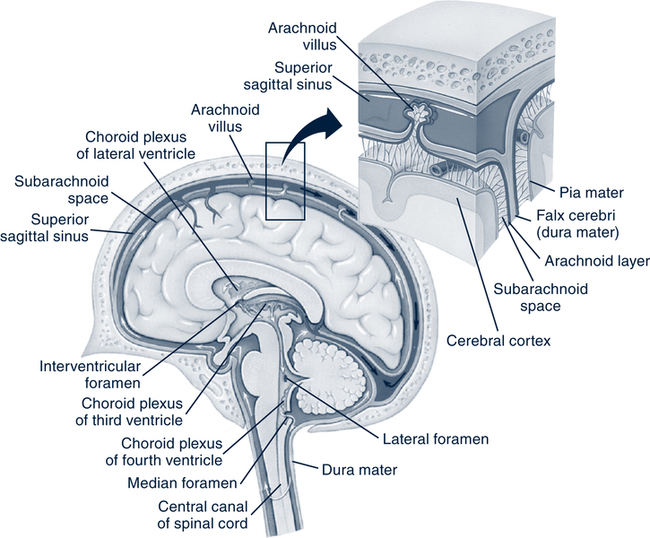
Cerebrospinal Fluid and Ventricular System
Hippocampus and the Hypothalamic–Pituitary–Adrenal Axis
Cerebrospinal Fluid
Balance and Motor Function
Reticular Formation and Sleep Patterns
Sensorimotor Function
Assessment of Cognitive Function
Selected Cognitive Function Screening Instruments
Functional Assessment
Mental Status Examination
Depression Assessment
Cognitive Disorders Associated with Altered Thought Processes
Depression
Clinical Manifestations
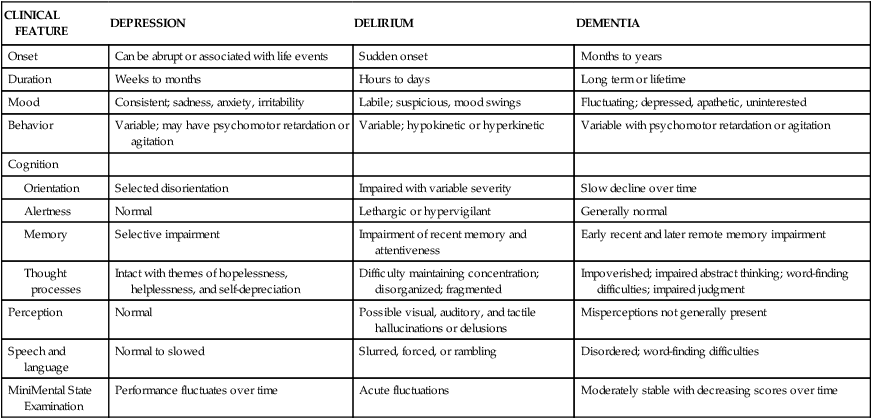
CLINICAL FEATURE
DEPRESSION
DELIRIUM
DEMENTIA
Onset
Can be abrupt or associated with life events
Sudden onset
Months to years
Duration
Weeks to months
Hours to days
Long term or lifetime
Mood
Consistent; sadness, anxiety, irritability
Labile; suspicious, mood swings
Fluctuating; depressed, apathetic, uninterested
Behavior
Variable; may have psychomotor retardation or agitation
Variable; hypokinetic or hyperkinetic
Variable with psychomotor retardation or agitation
Cognition
Selected disorientation
Impaired with variable severity
Slow decline over time
Normal
Lethargic or hypervigilant
Generally normal
Selective impairment
Impairment of recent memory and attentiveness
Early recent and later remote memory impairment
Intact with themes of hopelessness, helplessness, and self-depreciation
Difficulty maintaining concentration; disorganized; fragmented
Impoverished; impaired abstract thinking; word-finding difficulties; impaired judgment
Perception
Normal
Possible visual, auditory, and tactile hallucinations or delusions
Misperceptions not generally present
Speech and language
Normal to slowed
Slurred, forced, or rambling
Disordered; word-finding difficulties
MiniMental State Examination
Performance fluctuates over time
Acute fluctuations
Moderately stable with decreasing scores over time
Delirium
Risk Factors
Management
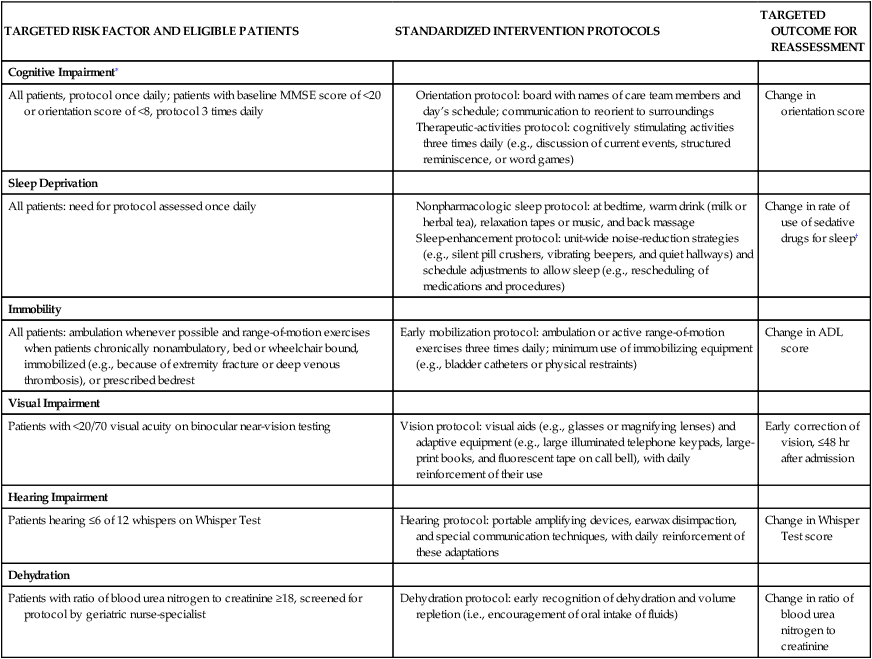
TARGETED RISK FACTOR AND ELIGIBLE PATIENTS
STANDARDIZED INTERVENTION PROTOCOLS
TARGETED OUTCOME FOR REASSESSMENT
Cognitive Impairment∗
All patients, protocol once daily; patients with baseline MMSE score of <20 or orientation score of <8, protocol 3 times daily
Change in orientation score
Sleep Deprivation
All patients: need for protocol assessed once daily
Change in rate of use of sedative drugs for sleep†
Immobility
All patients: ambulation whenever possible and range-of-motion exercises when patients chronically nonambulatory, bed or wheelchair bound, immobilized (e.g., because of extremity fracture or deep venous thrombosis), or prescribed bedrest
Early mobilization protocol: ambulation or active range-of-motion exercises three times daily; minimum use of immobilizing equipment (e.g., bladder catheters or physical restraints)
Change in ADL score
Visual Impairment
Patients with <20/70 visual acuity on binocular near-vision testing
Vision protocol: visual aids (e.g., glasses or magnifying lenses) and adaptive equipment (e.g., large illuminated telephone keypads, large-print books, and fluorescent tape on call bell), with daily reinforcement of their use
Early correction of vision, ≤48 hr after admission
Hearing Impairment
Patients hearing ≤6 of 12 whispers on Whisper Test
Hearing protocol: portable amplifying devices, earwax disimpaction, and special communication techniques, with daily reinforcement of these adaptations
Change in Whisper Test score
Dehydration
Patients with ratio of blood urea nitrogen to creatinine ≥18, screened for protocol by geriatric nurse-specialist
Dehydration protocol: early recognition of dehydration and volume repletion (i.e., encouragement of oral intake of fluids)
Change in ratio of blood urea nitrogen to creatinine
Dementia
Reversible Dementia
Alzheimer’s Disease
Risk Factors
Genetic Factors
Environmental Factors
Nutritional Factors
Diagnostic Studies
Treatment
Vascular Dementia
Treatment
Lewy Body Dementia
Management
Frontotemporal Dementia
Clinical Manifestations
Other Dementia-Related Diseases
Normal Pressure Hydrocephalus
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cognitive and Neurologic Function
Get Clinical Tree app for offline access