Chapter 19. CNS 2. Epilepsy and Parkinson’s disease
Epilepsy247
Introduction 247
Causes of seizures 248
Drugs that may cause seizures 248
Terms used to describe seizures 248
Types of epilepsy 248
General mechanistic approach to the treatment of epilepsy 249
The aims of treatment 249
Well-established drugs used in tonic–clonic and partial seizures 249
Newer drugs used in tonic–clonic and partial seizures 251
Outcome of treatment 254
Drugs used in absence seizures 254
Fatal adverse reactions in children 254
Driving 254
Drug combinations 254
Status epilepticus 254
Febrile convulsions 255
Antiepileptics and pregnancy 255
Prognosis in epilepsy 256
Summary256
At the end of this chapter, the reader should be able to:
• give an account of the different age-related causes of epilepsy and list the three main approaches to the treatment of epilepsy with drugs
• provide examples of well-established and newer drugs and their adverse effects and contraindications and appreciate the importance of phenytoin monitoring
• describe the treatment of absence seizures with drugs and describe the use of drugs to treat status epilepticus
• explain how to treat febrile convulsions and about reassuring parents
• give an account of antiepileptic drugs, pregnancy and eclampsia
• discuss the epileptic patient’s special needs and problems, e.g. driving
• give the two main approaches to the treatment of Parkinson’s disease with drugs and be able to give examples
• give an account of the problems associated with the use of levodopa and other drugs
Epilepsy
Introduction
The word epilepsy is derived from Late Latin epilepsia, from the Greek e pilambanein, which means to seize or attack. Hippocrates wrote about epilepsy in about 400 bc, calling it the ‘sacred disease’, since people experiencing seizures were assumed to be possessed by or communicating with the gods. These days, although much more is known about epilepsy, it is still not fully understood and it is still incurable.
Epilepsy affects at least 350 000 people in the UK. About 30 000 people develop epilepsy every year and epilepsy affects about 1 in 20 people at some time during their lives. Many studies have found a slightly higher incidence among men.
Causes of seizures
Some causes (see also Michael 1999a) of epileptic seizures are summarized below.
Neonatal onset
• Congenital brain malformation
• Asphyxia or hypoxia or intracranial trauma during delivery
• Infection
• Intracranial haemorrhage
• Electrolyte or metabolic disturbances.
Childhood and adolescence
• Brain tumours or trauma
• Cerebral degenerative disease or cerebral palsy
• Congenital brain malformation
• Febrile convulsions (see below)
• Chemical toxicity, e.g. lead, drugs
• Hereditary, e.g. tuberous sclerosis
• Hydrocephalus
• Idiopathic
• Infection
• Lennox–Gastaut syndrome (see below)
• Other diseases, e.g. renal disease.
Adult
• Birth trauma
• Brain trauma or tumours
• Cerebral degenerative disease
• Cerebral vascular disease, e.g. infarction
• Congenital disease
• Drug toxicity, drug abuse and withdrawal, including alcohol
• Idiopathic
• Metabolic disturbances.
Clearly, there is overlap among the various age groups.
Drugs that may cause seizures
• Anaesthetics, e.g. enflurane, halothane, ketamine
• Antibiotics, e.g. amphotericin, cephalosporins, chloroquine, cycloserine, fluconazole, isoniazid, penicillins
• Antidepressants and antipsychotic drugs, e.g. baclofen, cocaine, lithium, tricyclics
• Cardiovascular drugs, e.g. intravenous lidocaine, procaine
• Endocrine drugs, e.g. desmopressin, insulin, oxytocin, prednisolone
• Radiographic contrast media, e.g. certain meglumine derivatives, metrizamide
• Stimulant drugs, e.g. aminophylline, caffeine, theophylline.
This list is far from comprehensive, but alerts the reader to the fact that many drugs are capable of producing seizures in some patients.
Terms used to describe seizures
The following terms are used:
• focal
• petit mal (absence seizures)
• grand mal (tonic–clonic)
• psychomotor epilepsy
• partial seizures
• generalized seizures.
Types of epilepsy
There are several varieties of epilepsy and they vary in their response to drugs. In focal epilepsy the attack arises from a focal electrical discharge in the brain. This may produce a brief aura, which is a feeling or movement. If the discharge becomes generalized, the patient falls unconscious and passes through the typical tonic and clonic phases, regaining consciousness after a varying interval. This is known as a tonic–clonic (grand mal) seizure. Sometimes the spread of the discharge is limited ( partial seizure), producing psychological disturbances ( psychomotor seizure) or various involuntary movements, but without loss of consciousness. When a seizure starts as a partial seizure and then spreads to become generalized, this is often referred to in the literature as ‘secondary generalizations’. In some patients, mainly children, the electrical discharge is widespread from the start, and causes an absence (petit mal) seizure, which is a brief interference with consciousness. The objective in treating epilepsy is to abolish the attacks completely by means of drugs.
Readers will encounter the terms myoclonus and myoclonic jerks when reading about epilepsy. These terms refer to the sudden jerking of the limbs that occurs in patients with epilepsy and in those with degenerative neurological disease. Some healthy people experience nocturnal myoclonic jerks when falling asleep.
General mechanistic approach to the treatment of epilepsy
Currently used drugs aim to control epilepsy through one of three main mechanisms:
• enhancement of the activity of the inhibitory brain neurotransmitter gamma-aminobutyric acid (GABA)
• inhibition of the activity of the excitatory brain neurotransmitter glutamate
• directly blocking sodium and/or calcium channels in the nerve cell membrane.
Some drugs, such as gabapentin (see below), are effective but the mechanism is still not well understood.
The aims of treatment
• The maintenance of as normal a lifestyle as possible for the patient
• Prevention of occurrence of seizures through maintenance of adequate blood levels of antiepileptic drugs
• The use of single or combination drug therapy using careful grading of drug dosage
• Choice of drugs and dosage frequency to optimize patient compliance
• Regular monitoring for drug toxicity
• Regular monitoring of patient status regarding drug interactions.
Although there are now a number of drugs that are useful in controlling epilepsy, it is usually best to start treatment with one drug (called ‘monotherapy’) and use multiple drug regimens only in resistant cases. The initial dose should be low and it should be increased until control of the seizures is achieved or adverse effects develop.
Well-established drugs used in tonic–clonic and partial seizures
For more information, see also Michael 1999b.
Phenytoin
Phenytoin is a member of the hydantoin group of compounds, which are structurally related to barbiturates such as phenobarbital (see below).
Mechanism of action
Phenytoin sodium appears to act by blocking sodium channels in nerve membranes. This reduces the excitability of nerve cells and prevents the abnormal discharge from spreading in the brain.
Therapeutic use
Phenytoin is well absorbed by mouth and does not produce drowsiness or sleep. Its effectiveness as an anticonvulsant and the incidence of side-effects depend largely on the plasma level of the drug. Finding the correct dose may be difficult for several reasons:
• Patients vary considerably in the rate at which they break down phenytoin, so there is a wide variation of dose requirements between patients.
• The relationship between dose and plasma level is not linear; this means that a small increase in the dose may cause a considerable rise in the plasma level (Fig. 19.1).
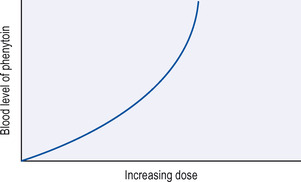 |
| Figure 19.1 Relationship between dosage and blood level of phenytoin. |
• Because phenytoin is slowly broken down, once the daily dosage is adequate, it takes about a week for the plasma level to become steady. Therefore, the dose should not be altered at less than fortnightly intervals.
Adverse effects
These are fairly common with phenytoin and include:
• greasy skin and hirsutism may cause problems in women
• macrocytic anaemia due to folic acid deficiency
• gum hypertrophy; dental care is important
• lymph node enlargement
• a variety of rashes.
Drug interactions
These are common and underline the need for regular measurement of plasma levels of phenytoin:
• Phenytoin is largely bound to plasma proteins in the blood, and can be displaced from these proteins by other drugs such as sodium valproate, which is another antiepileptic drug (see below), and by aspirin. This will increase the concentration of free phenytoin in the blood and therefore effectively increase the dose. Increasing the unbound fraction of phenytoin in the blood also increases the amount of phenytoin that can be metabolized in the liver, and all this results in highly unpredictable levels of phenytoin in the circulation.
• Phenytoin induces liver enzymes that metabolize drugs such as hydrocortisone, oral contraceptives, theophylline, tricyclic antidepressants and thyroxine. among others. This will decrease the efficacy of those drugs.
Patient compliance and phenytoin monitoring
As already mentioned above, it is necessary to measure plasma levels of phenytoin regularly. This is necessary not only because of phenytoin’s interactions with other drugs and because of the non-linear relationship between dose and plasma levels but also because patients, especially elderly patients, may not be taking the drug as prescribed.
Fosphenytoin, which is a prodrug that is converted into phenytoin in the body after injection, has been introduced, and is also mentioned below in the following (newer drugs) section.
Carbamazepine
Carbamazepine is a drug that is chemically related to the tricyclic antidepressants. It is believed to act by blocking sodium channels in the nerve membrane and keeping the conducting nerve in an inactive state.
Therapeutic use
Carbamazepine is widely used in the control of tonic–clonic and partial seizures.It is also used to relieve the pain of trigeminal neuralgia and in the treatment of bipolar depression (see p. 285). Carbamazepine is given orally and is fairly slowly absorbed from the intestine. Its rate of breakdown in the body increases with prolonged use, because, like phenytoin, it induces the liver enzymes that break it down. Estimation of plasma levels may help to determine the correct dose, although the correct dose is more usually decided on by the patient’s response than the plasma levels of the drug. Children may break down the drug rapidly, so they may require three or four doses daily. The drug is introduced at a lower dose and this is gradually increased over a month. There is a slow-release or ‘retard’ formulation of carbamazepine that may be used at higher dosages, and also a suppository for when the oral route is not feasible.
Adverse effects
Up to one-third of patients who take carbamazepine experience adverse effects, but only about 5%of patients have to discontinue treatment. The most common adverse effects include rashes, dizziness and drowsiness, blurring of vision, depression of the leucocytes of the blood, and, occasionally, jaundice and excessive salivary secretion. At higher doses, carbamazepine can have an antidiuretic effect and cause dyskinesia, photosensitivity and arrhythmias. It should be used with caution in cases of renal failure and the dose should be reduced in patients with liver disease.
Drug interactions
These occur with warfarin and erythromycin.
Phenobarbital
Phenobarbital, the oldest drug in use for epilepsy, is a barbiturate. Phenobarbital reduces the spread of electrical excitation in the brain in several ways, but its principal action is by enhancing the action of the inhibitory neurotransmitter GABA by binding to sites on the GABA receptor. It also inhibits the action of the excitatory neurotransmitter glutamate.
Therapeutic use
Phenobarbital is not the drug of choice in epilepsy, because of its potential neurotoxicity. It is generally prescribed only when patients cannot tolerate other drugs. It is, however, still widely used in developing countries, because of its low cost.
Phenobarbital is slowly absorbed. The major portion is broken down in the body and the rest slowly excreted by the kidneys. Its action is therefore prolonged over about 12 hours. Phenobarbital is particularly effective in the treatment of tonic–clonic seizures, but may also be used in other types of epilepsy. It may be used as an alternative to phenytoin in the treatment of status epilepticus (see below).
Adverse effects
They are not uncommon: drowsiness and ataxia may be troublesome and occasionally a rash resembling measles is seen. Phenobarbital, like phenytoin, is a powerful inducer of enzymes in the liver, particularly those that break down other drugs. For example, phenobarbital increases the rate of breakdown of anticoagulants and of the estrogens, which are used in oral contraceptives, and whose effects may therefore be reduced. In adults, the drug may cause sedation and mood changes, especially depression. In children, however, the reverse occurs and the drug may produce hyperactivity, aggression and insomnia. Phenobarbital can cause osteomalacia through vitamin D deficiency and megaloblastic anaemia through folic acid deficiency. Like other barbiturates, phenobarbital causes physical dependence (see also p. 293), and sudden cessation of treatment may precipitate serious withdrawal symptoms, including sometimes-fatal convulsions, especially in the elderly.
Primidone
Therapeutic use
Primidone is in many ways similar to phenobarbital and is effective against tonic–clonic attacks. It is important to start treatment with a low dosage and gradually increase the dose, otherwise adverse effects such as drowsiness, vertigo and vomiting may occur. It should not be combined with phenobarbital.
Sodium valproate and valproic acid
Sodium valproate has been available for many years, being introduced in the early 1970s. Valproic acid was introduced in the 1990s.
Mechanism of action
Sodium valproate has several CNS actions. It maintains levels of GABA after the neurotransmitter has been released, by inhibiting enzymes that break it down, although it is not known if this is how it blocks convulsions. It also increases the breakdown of the excitatory neurotransmitter glutamate. Sodium valproate also has a weak blocking action on sodium channels in the nerve cell membrane.
Therapeutic use
Sodium valproate is well absorbed after oral administration and has a half-life in the circulation of about 15 hours. It is effective against both petit and grand mal epilepsy. It is especially useful for treating infants since it has relatively low toxicity and few sedative effects. It is also useful for treating older children who may suffer simultaneously with both petit and grand mal epilepsy.
Adverse effects
The drug is known to cause teratogenic effects such as spina bifida. Sodium valproate fairly commonly causes a modest fall in the platelet count. Occasionally this is severe and the patient should be warned to report any bruising or bleeding. It is advisable to carry out a platelet count before major surgery. Very rarely it causes serious liver damage, particularly in those with pre-existing liver disease or in children with congenital or accidental brain damage. Drowsiness, thinning of the hair (usually reversible) and weight gain are not uncommon.
Clonazepam and diazepam
Clonazepam and diazepam are benzodiazepine drugs. Diazepam ( Valium) is the most well known of this group of drugs. They may produce their antiepileptic effects by enhancing the inhibitory effect of GABA. They are effective in all forms of epilepsy but cause sedation. There is also a troublesome withdrawal syndrome associated with the benzodiazepines, which can worsen epileptic seizures if these drugs are stopped. They are most useful in treating status epilepticus (see later). A newer benzodiazepine, clobazam, has been introduced (see below). The benzodiazepine lorazepam is useful in status epilepticus (see below).
Newer drugs used in tonic–clonic and partial seizures
These newer drugs are still been evaluated (see also Michael 1999c).
Felbamate
Felbamate is chemically related to a much older anxiolytic drug called meprobamate, which is no longer in use. The mechanism of action of felbamate is not fully understood, although it is thought to act by enhancing GABA activity, inhibiting glutamate activity and blocking sodium channels.
Therapeutic use
Felbamate is effective over a wide range of epileptic conditions. It is particularly effective in the treatment of partial seizures – with or without secondary generalizations – that do not respond to other treatments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
