Allodynia: abnormal perception of pain from a normally nonpainful mechanical or thermal stimulus
Spinal cord tumors
Traumatic spinal injuries
Intervertebral disc disease/sciatica
Spinal stenosis with root compression
Compression of nerve plexuses
Thalamic pain syndrome
Metastatic bone pain (e.g., vertebra)
Cauda equina disease
Trigeminal neuralgia
Postherpetic neuralgia
Peripheral nerve diseases/entrapment syndromes
Neuropathics: mononeurotherapy, polyneuropathy, diabetic neuropathy
Complex regional pain syndromes I and II
Crushing nerve injuries
Phantom pain
Anesthesia dolorosa: pain in an area or region that is anesthetic
Central pain: pain associated with a primary lesion or dysfunction in the CNS
Complex regional pain syndrome type 1 (CRPS-I), previously called reflex sympathetic dystrophy: a syndrome of varying patterns that include pain that is out of proportion to injury (if it can even be identified), sensory abnormalities (burning pain and hyperpathia), focal autonomic abnormalities, motor abnormalities, and trophic changes; follows soft tissue or bone injury most often to the limbs, but without discernible nerve injury (Box 26-2)8, 9
CRPS-II, previously called causalgia: has a similar clinical presentation to CRPS-I, but there is related nerve injury
Deafferentation pain: pain due to loss of sensory input into the CNS, such as occurs with avulsion of the brachial plexus or other types of lesions of peripheral nerves or due to pathology of the CNS. Phantom limb pain is an extreme example of deafferentation pain.
Dysesthesia: an unpleasant abnormal sensation, whether spontaneous or evoked
Hyperpathia: abnormally painful reaction to a stimulus, especially a repetitive stimulus; pain is often explosive in nature
Hyperesthesia: an increased sensitivity to stimulation, excluding special senses
Hypoesthesia: a diminished sensitivity to stimulation, excluding special senses
Neuralgia: pain in distribution of a nerve or nerves
Neuritis: inflammation of a nerve or nerves
Neuropathy: a disturbance of function or pathological change in a nerve
Nociceptive neuron: a central or peripheral neuron of the somatosensory nervous system that is capable of encoding noxious stimuli
Nociceptive pain: pain that arises from actual or threatened damage to nonneural tissue and is due to activation of nociceptors.
Opioid-induced hyperalgesia (OIH): a state of nociceptive sensitization caused by opioid exposure. OIH is manifested by a paradoxical response in which opioids, administered for the treatment of pain, are capable of enhancing pain, sensitivity, and loss of analgesic efficacy. Although the precise mechanism of OIH is not understood, it is thought to result from neuroplastic changes in the peripheral and CNS that lead to sensitization of pronociceptive pathways. The N-methyl-D-aspartate (NMDA) receptor has been implicated in the pathophysiology of OIH.10
Description: severe, deep, burning, or lancinating pain that is out of proportion to the injury present and wide variability of symptoms; pathophysiology and treatment are unclear; diagnosis is based on clinical presentation.
Diagnostic criteria: from the International Association for the Study of Pain:
Follows a traumatic event to soft tissue or bone without an apparent nerve lesion (in 10% of patients, no source of trauma can be identified)
Spontaneous pain (hyperalgesia and allodynia) disproportionate to the precipitating event
From onset of precipitating event there is edema, skin, temperature changes (usually cool), and color changes in distal aspect of affected limb
No other explanation for the degree of pain and dysfunction
In the latter stages look for hair loss, brittle nails, and skin atrophy in affected limb and impaired motor function, including weakness, tremor, and ankylosis.
Treatment: physical therapy, drug therapy, or spinal cord stimulator (transcutaneous electrical nerve stimulation) or sympathectomy (sympathetic block) in patients who do not respond to physical therapy and drugs used for neuropathic pain. Drug therapy includes tricyclic antidepressants, anticonvulsants, corticosteroids, lidocaine, nonopioids, opioids, adjuvant analgesics, and alendronate.
Pain: an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage
Pain tolerance level: the maximum amount of pain that an individual is willing to accept in a given situation
Pain threshold: the minimum intensity of a stimulus that is perceived as painful
Paresthesia: an abnormal sensation, whether spontaneous or evoked
Peripheral neurogenic pain: pain caused by a primary lesion or dysfunction in the PNS
Sensitization: increased responsiveness of nociceptive neurons to their normal input, and/or recruitment of a response to normally subthreshold inputs. (central sensitization if occurs in CNS nociceptive neurons; peripheral sensitization if occurs in peripheral nociceptive neurons)
Trigger point: a hyperirritable area or site in muscle or connective tissue, tender when compressed, usually associated with myofascial pain syndromes.11
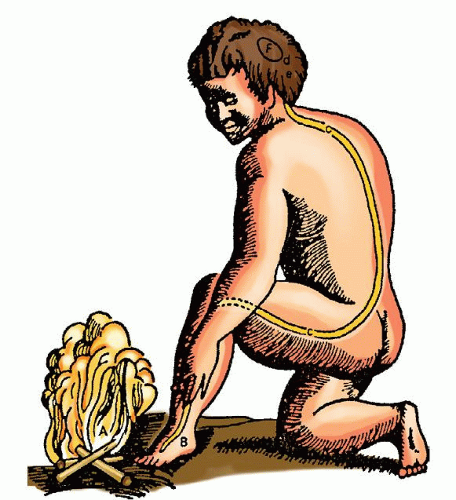
center of all sensation and that pain was associated with demons and gods. It was not until the 17th century that Descartes described some of the beginning concepts of pain pathways. According to Descartes, when the foot comes near a fire, the sensation enters the body and travels up threads to the brain (Fig. 26-1).
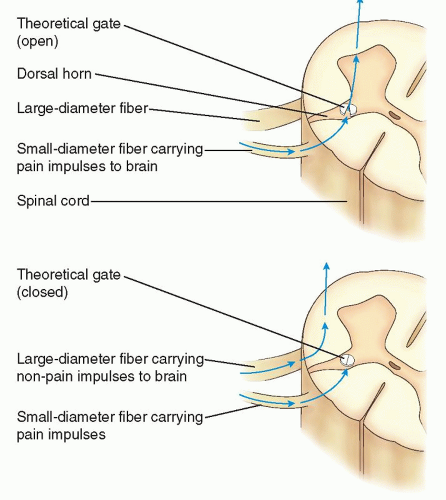
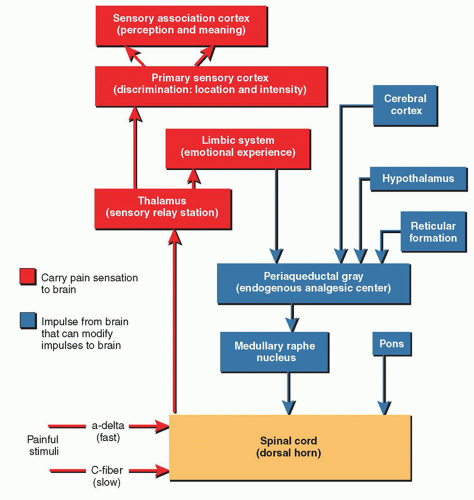
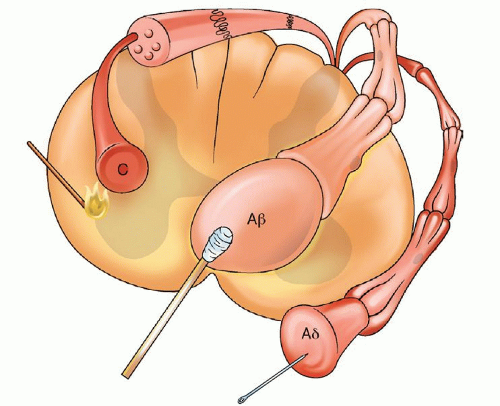
to generate an action potential. Pain impulses enter the spinal cord through the dorsal root synapse in lamina I, II, or V and then cross the other side of the spinal cord through the gray matter to the spinothalamic tract. Next, the process of perception, which involves both cortical and limbic system structures occurs. The spinothalamic tract transmits pain impulses to the thalamus and then to the primary sensory cortex, where the discrimination of pain is perceived, and then to the limbic cortical areas for perception of the affective and emotional aspects of pain. The final process, modulation, occurs at multiple levels (e.g., peripheral, spinal, and supraspinal). Nerve fibers in the descending inhibitory pathways release inhibitory substances (endogenous opioids, serotonin, norepinephrine, GABA) at synapses with other neurons in the dorsal horn which block nociceptive transmission. The pain impulse also activates a reflex withdrawal from the painful stimulus in the spinal cord, and also increases the state of arousal and emotional, autonomic, and neurohumoral responses.1
and improve overall quality of life. Through medical management, graded physical exercise, and cognitive and behavioral pain and stress management techniques, the following outcomes have been reported: reductions in pain scores, reduced emotional distress, decreased opioid use, improved function, and reduced health care utilization.21 (Schatman in Bonica)
TABLE 26-1 COMMON COMPONENTS OF AN INTEGRATED INTERDISCIPLINARY REHABILITATION PROGRAM | |
|---|---|
|
TABLE 26-2 COMMON PSYCHOLOGICAL AND BEHAVIORAL TECHNIQUES USED TO TREAT CHRONIC NONCANCER PAIN | |
|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree