Chapter 25. Caring for children and young people with body fluid and electrolyte imbalance
Agnes B. Kanneh
ABSTRACT
Ensuring adequate hydration and electrolyte balance are essential aspects of care for children and young people in both health and disease. Balance in volume and solute composition of body fluids and electrolytes is ensured by the well-coordinated and integrated action of the homeostatic organs, carefully matching intake with output. Body fluids and electrolytes balance relates to equilibrium between intake and output, or between gain and loss in maintaining homeostasis. Imbalances occur when the body is deficient in, or has excess of the required amounts that ensure health and well-being. Recurrent imbalances in body fluids and electrolytes can have long-term adverse effects on the child’s physical and cognitive growth and development (Petri et al 2008). This is because explanations offered on the structure and biological functions of water affirm the notion that water is the chemical of life, second only to oxygen (Eastwood 1997, Martini Nath, 2009).
The availability of and access to safe drinking water is a basic human right and, water has been suggested as a neglected nutrient in the young child (Bourne et al 2007).
In any human environment, safe water and electrolyte intake in the form of drinking is necessary to ensure a balance that maintains effective organ system function. Here, children – and especially the very young – are dependent on their parents and carers, including healthcare professionals, to meet this fundamental human need because of limited self-care ability. On an annual global scale, pneumonia and diarrhoea account for about 10 million deaths in children less than 5 years of age. Sadly, large numbers of children under 5 years of age worldwide present with acute gastroenteritis annually, causing nearly 2 million deaths. In the UK, 204 of every 1000 GP consultations and 7 of every 1000 hospital admissions of children under 5 years are due to gastroenteritis. Thus, acute diarrhoea is the leading cause of body fluid and electrolyte imbalance in children worldwide (Dalby-Payne & Elliott 2008).
LEARNING OUTCOMES
• Review key functions of water and electrolytes in children and young people.
• Identify and examine possible causes of body fluid and electrolyte imbalance in children and young people.
• Detail the regulatory systems that maintain body fluid and electrolyte balance.
• Specify common causes of body fluid and electrolyte imbalance in children and young people.
• Explain the clinical manifestations of dehydration and electrolyte imbalance in children and young people.
• Detail the principles of nursing care and evidence-based practice for the child who presents with body fluid and electrolyte imbalance caused by acute diarrhoea and vomiting.
Water composition of the body
Body fluids
The cell is the fundamental unit of life and metabolism and an adequate intake of water is essential to the survival and everyday activities of the metabolically active growing and developing child in both health and disease. Body fluids comprise total body water (TBW), with its dissolved chemical particles. Throughout life a large proportion of the body is water, changing as age progresses (Fig 25.1).
 |
| Fig. 25.1 The percentage of total body water from age 1 year |
Water is vital to the existence of all living organisms and is the most abundant molecule of their cell mass (Campbell & Reece 2008). Two-thirds of the total body weight is water and it is the body’s single most important constituent (Martini & Nath, 2009). Table 25.1 shows the water composition of various tissues in the average individual. In the absence of water, chemical reactions, metabolic processes and regulatory systems become severely compromised and eventually cease to function (Tortora & Derrickson 2009). A healthy adult can survive for around 10 days without water; a healthy child will survive only 3–4 days without water. For a thorough comprehension of the value of water to life, growth and development, and hence the deleterious effects of uncorrected body fluid and electrolyte imbalance, an understanding of the chemical and physical properties of water is essential (see Chapter 27 in Martini Nath, 2009). Daily fluid maintenance requirements take into consideration losses from the skin, airways, gastrointestinal tract and urine.
| Tissue | Percentage of water | Litres of water | Percentage of total body water |
|---|---|---|---|
| Skin | 72 | 9.72 | 22 |
| Skeleton | 22 | 2.47 | 5 |
| Blood | 83 | 3.11 | 7 |
| Adipose | 10 | 0.90 | 2 |
| Muscle | 76 | 24.51 | 55 |
Body fluid compartments
TBW in multicellular human organisms is functionally divided into compartments. These are specific enclosures in which the fluid exists in relation to cell anatomy, with the cell membrane and capillary endothelum forming the interface between the compartments. Body fluid compartments differ in both their volume and solute composition. The solute composition, because of the osmotic movement of water, is in equilibrium between the extracellular fluid and intracellular space. Specific composition of each compartment establishes the optimal environment for the biochemical reactions that occur within them. The absolute and relative size and volume of each compartment varies with the age and sex of the individual. In relation to tissue cells, TBW exists in two main compartments and a third minor compartment:
• Intracellular fluid (ICF) compartment: comprises the fluid existing inside tissue cells.
• Extracellular fluid (ECF) compartment: comprises body fluid existing outside and between tissue cells.
• Transcellular fluid: an extra small compartment.
The body of the average adult contains approximately 42 L of water (Fig. 25.2), which is divided into the various body fluid compartments (Guyton & Hall 2005). Figure 25.2 and Table 25.2 illustrate the distribution of body water within the compartments and Fig. 25.3 and Fig. 25.4 apply specifically in the various imbalances in the child, where fluid shifts can occur, thereby changing the osmolality of fluids within the three compartments.
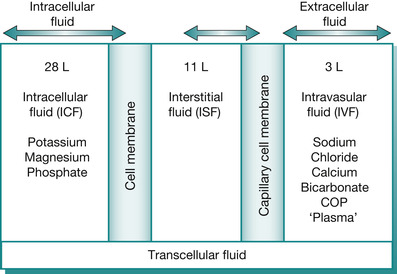 |
| Fig. 25.2 Distribution of total body water within compartments in the average adult; showing all the body fluid compartments and their corresponding constituents. The intravascular fluid (IVS) is the effective circulating blood volume (CBV), maintained largely with the help of the plasma proteins, with albumin maintaining 70–80% of the colloid oncotic pressure (COP). Colloid solutions with the same COP as plasma will stay in the IVS and thus are most efficient in restoring the CBV in hypovolaemia. Alternatively, crystalloid solutions administered intravenously are isotonic to plasma and thus distributed to the extracellular fluid (ECF), with the majority of it to the interstitial space (ISS). The sodium/potassium (Na/K) ATPase pump actively pumps sodium into the extracellular space (ECS) and potassium into the intracellular space (ICS), although glucose-containing fluids are evenly distributed in the ICS to rehydrate cells |
| ECF, extracellular fluid; ICF, intracellular fluid; TBW, total body water. | |||
| Age | TBW (%) | ECF (%) | ICF (%) |
|---|---|---|---|
| Preterm neonate | 80 | 45 | 35 |
| Full-term neonate | 75 | 40 | 35 |
| 1–12 months | 65 | 30 | 35 |
| 1–12 years | 60 | 20 | 40 |
| Adult | |||
| male | 55 | 25 | 30 |
| female | 50 | 20 | 30 |
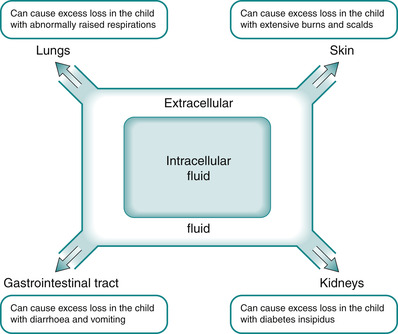 |
| Fig. 25.3 The relationship between intracellular fluid, extracellular fluid and exchange with the external environment within the body. |
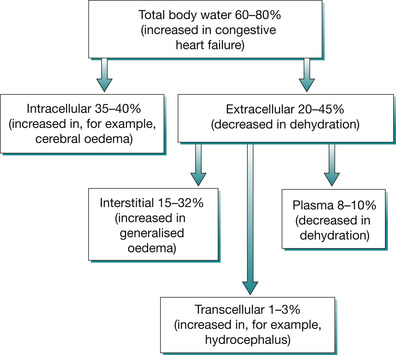 |
| Fig. 25.4 Total body water composition in children in health and disease. |
Intracellular fluid
Intracellular fluid comprises the body water that exists inside the tissue cells that make up the child’s body, and accounts for 35–40% of the total body water. The intracellular fluid is contained within a closed space (the cell) so it is not easy to estimate and there is a limit to which it can expand without causing stress to the cell membrane. For this reason, pure water (free water) is not administered intravenously because it is hypotonic relative to the intracellular solute composition.
Theoretical intravenous administration of pure water causes the cells (e.g. erythrocytes) to exist in a hypotonic environment relative to their intracellular space. Water will then move by the process of osmosis into the erythrocytes, causing them to swell until their cell membrane can no longer accommodate the increase in cell volume, causing them to burst (cell lysis). A similar situation will occur in water intoxication or overhydration with acute hyponatraemia. Here, the cells most vulnerable to osmotic fluid shift into their intracellular compartment are brain cells. Ultimately, cytotoxic cerebral oedema, with its attendant raised intracranial pressure (ICP), occurs. Cerebral function is altered, and this is reflected in an abnormal score on the Glasgow Coma Scale. Unrelieved, cerebral neuropathy resulting from the increased ICP can cause seizures, coma and death. There is increasing evidence to show that iatrogenic (treatment – induced) hyponatraemia is a real clinical threat to children especially in the postoperative period and the high-dependency setting. The only saving grace in preventing adverse outcomes in these circumstances is the careful monitoring of serum sodium levels in the children (Au et al 2008).
Extracellular fluid (Ichikawa 1990, Martini Nath, 2009)
Extracellular fluid exists (ECF) outside and between tissue cells, supporting and nourishing them as they bathe in it. ECF accounts for 20–45% of the total body weight in children and is further subdivided into two subcompartments:
• Intravascular fluid compartment – inside blood vessels
• Interstitial fluid – in between tissue cells.
Extracellular fluid is fundamental to the regulation and balance of body fluids and electrolytes because it is the changes in this compartment that act as a trigger for the activation of regulatory systems in the quest to maintain homeostasis.
Intravascular fluid compartment
The components in the intravascular fluid compartment enable the cardiovascular system to meet the metabolic needs of the body.
The cardiovascular system is a continuous, closed, blood-filled circuit equipped with a muscular and electrical pump (the heart) that maintains the pulmonary and systemic circulation. The cardiovascular system consists of:
• the heart: an electrical muscular pump that establishes the pressure gradient that ensures effective blood flow to tissue cells
• blood vessels: the channels or passageways through which blood flows within the body
In plasma volume deficit, hypovolaemia occurs, increases blood viscosity and the packed cell volume, which can increase the propensity for thromboembolic episodes in a child with nephrotic syndrome, for example.
The intravascular fluid comprises plasma and makes up 8–10% of the total body water. It represents the entire amount of fluid inside the blood vessels, minus that inside the blood cells. The intravascular space is bounded by the capillary endothelial cell membrane. Plasma, the suspended blood cells and dissolved particles constitute the circulating blood volume (CBV). This is the actual fluid volume perfusing the tissue cells of the vital organs. It is in contact with and stimulates the volume receptors and baroreceptors in the vena cavae and aorta, respectively. Plasma, the fluid component of blood, contains salts, organic and inorganic compounds and hormones. Serum is plasma minus the clotting factors, in particular fibrinogen.
Interstitial fluid (Tortora & Derrickson 2009, Martini Nath, 2009)
The interstitial fluid (ISF) comprises the fluid interposed between the intravascular space and intracellular space. The interstitial fluid is the fluid between the cells, i.e. it bathes the cells. Nutrients and respiratory gases, plus enzymes, hormones and other substances, reach tissue cells via the arterial ends of the capillaries. Cells need to be cleared of their metabolic waste products and this is a function of the venous ends of capillaries; the process enables the waste products to reach their designated depots, such as the kidneys and lungs, for disposal.
ISF is tissue fluid and the interstitial space can be viewed as a ‘biological bureau de change’. Effective distribution of fluid between the intravascular and interstitial compartments is determined by the difference between the hydrostatic pressure, which is generated by myocardial contractility and arteriolar vasoconstriction, and the opposing plasma oncotic pressure, generated by plasma proteins. This partially explains oedema in the child with nephrotic syndrome who becomes hypoproteinaemic.
Lymph, the fluid that circulates in the lymphatic vessels, is part of the interstitial fluid. It is an important fluid because it reclaims the small amounts of fluid that remains in the interstitial space back into the intravascular space, thus preventing fluid backlog leading to oedema. Although body fluids are compartmentalised, they are all in communication with one another with the help of their respective biophysical forces. Therefore fluid shifts occur in dehydration because fluid will tend to move into the compartment with the deficit.
 Activity
Activity
 Activity
ActivityCritical reflection on theory
With reference to the descriptions above, explain why a dehydrated child might manifest abnormal physical features of the skin and mucous membranes.
Transcellular fluid (Tortora & Derrickson 2009, Martini Nath, 2009)
Transcellular fluid is a minor part of the total body water, forming 1–3%. Its function is to lubricate and provide smooth movement between two closely related layers or membranes, e.g. the pleural membranes. In terms of the circulating blood volume, transcellular fluid is non-functional or vestigial. This is because it is not readily available to support the circulating blood volume in times of need, as in hypovolaemia for example. Transcellular fluid can, however, act as a third-space fluid in pathological conditions that cause accumulation of fluid in this space. Transcellular fluid comprises:
• cerebrospinal fluid (CSF) (excess = hydrocephalus)
• pericardial fluid (excess = pericardial effusion)
• pleural fluid (excess = pleural effusion)
• synovial fluid (excess = swollen joints)
• intraocular fluid (excess = glaucoma).
Biological functions of water
Essential functions of water in children and young people include:
• maintaining an adequate and effective CBV
• providing the aqueous medium for chemical reactions
• transporting vital metabolites within the body
• removing metabolic wastes and toxic materials from the body via their excretory depots
• ensuring effective thermoregulation
• maintaining balance in blood and other body fluids chemistry
• lubricating layers in close association and preventing friction on their movement
• supporting, cushioning and protecting body organs/structures.
These vital functions highlight the critical role adequate hydration plays in the effective functioning of the growing and developing child’s body at any age, particularly infancy. Table 25.3 explains why children, especially the very young, are at increased risk of body fluid and electrolyte imbalance. Also, the organ systems that maintain water balance are immature in children, so young children in particular are at risk of body fluid and electrolyte imbalance. The well-hydrated child should therefore feel warm, have pink and moist mucous membranes, vital signs that are normal for their respective age band and pass a minimum of 1 mL of urine per kilogram of body weight per hour (Table 25.4).
| ECF, extracellular fluid. | |
| Concept | Rationale(s) |
|---|---|
| Higher ratio of extracellular fluid volume to that of intracellular fluid volume | Blood plasma forms part of the ECF and it is this body fluid that is in direct contact with the environment, e.g. perfusing the subcutaneous capillaries underneath the skin. The close encounter of ECF with the environment thus means a higher turnover rate of ECF in the child than the adult |
| Children have a large surface area-to-volume ratio meaning a higher insensible fluid loss from the skin and airways | The surface area-to-volume ratio of a small child is greater than that of an adult. This increases water loss from the child through the skin in heat loss by radiation to the environment. The concept thus adds to the child’s increased biological need for water |
| Children have a higher metabolic rate | Children are highly metabolic individuals because of their ongoing growth and development, as well as their other life activities. Children therefore have a higher metabolic rate than adults, which in turn means that they proportionately require more water for both intercellular and intracellular synthesis and excretion of metabolic wastes |
| Children have a high rate of heat exchange | The high metabolic rate in the child means an increased energy expenditure, including thermal energy, thus a high rate of heat exchange. Water is required to facilitate this heat exchange, which increases the day-to-day water requirement for the child as compared with the adult |
| Children are not miniature adults | The homeostatic organs of children, especially infants, are immature and the control centre/brain (and their interconnecting pathways) require time to attain functional competence. Even the interaction between hormones and their target organ receptors takes time to mature in children. Therefore, the respective gains of control are less effective and at times exaggerated, as in the case of peripheral vasoconstriction in hypovolaemia. Children’s homeostatic systems are thus not very well able to respond and make the necessary adjustments in sudden and extreme changes in set-points. Finally, the self-care abilities in particular in infants are limited resulting in a total dependence on parents/carers |
| Weight (kg) | Fluid requirement |
|---|---|
| 2.5–6 kg | 150 mL/kg/24 hours |
| 6–10 kg | 120 mL/kg/24 hours |
| 10–20 kg | 100 mL/kg/24 hours |
| 20 kg and over | 75 mL/kg/24 hours |
The main electrolytes, their functions and regulatory systems
Electrolytes
Electrolytes/ions are inorganic molecules that, when dissolved in water, separate into their respective electrical charges: positive (cations) and negative (anions). It is essential that the positively charged ions are in balance with the negatively charged ions. The physical and chemical properties of electrolytes and their existence in specific cellular compartments just as water enable them to carry out their respective biological roles in the body of the growing and developing child: maintaining total body water, acid–base balance, energy balance, effective enzyme function, the creation of muscle and nerve cell membrane potentials. Also, electrolytes composition of plasma is vital for effective functioning of tissue cells, in particular those of excitable form, i.e. neurons and myocytes. Key electrolytes in the body and their chemical symbols are shown in Table 25.5.
| Cations | Anions |
|---|---|
| Sodium (Na +) | Chloride (Cl −) |
| Potassium (K +) | Bicarbonate (HCO 3−) |
| Calcium (Ca ++) | Phosphate (HPO 42−) |
| Magnesium (Mg ++) | ‘Proteins/amino acids’ (A −) Organic acids |
Most of the essential biological processes are dependent on electrolyte balance and affected by imbalance, which cannot be tolerated for a long time. Sodium and potassium are the chief positively charged ions. Sodium, potassium, chloride and bicarbonate are most likely to show in electrolyte imbalance. Sodium is the main extracellular cation and determines it’s osmolality and CBV. Potassium is the main intracellular cation and determines intracellular fluid volume and cell membrance potential.
The high metabolic rate and the demands of growth and developmental of the child in health and disease make both electrolytes and water critical to their biological well-being. Table 25.6 shows the daily electrolyte requirements in children and Table 25.7 shows the normal reference values for electrolytes in children. These figures are biologically determined reference values but can vary in different clinical conditions on an individual basis (Ichikawa 1990, Somers & Harmon 1999, Tortora & Derrickson 2009).
| Electrolytes | |
|---|---|
| Sodium | 1–3 mmol/kg/day |
| Potassium | 1–3 mmol/kg/day |
| Chloride | 2–3 mmol/kg/day |
| Calcium | 0.7–1.5 mmol/kg/day |
| Phosphorus | 0.9–1.5 mmol/kg/day |
| Electrolyte | Childhood reference value | Neonatal reference value |
|---|---|---|
| Sodium | 135–145 mmol/L | 132–145 mmol/L |
| Chloride | 100–106 mmol/L | 90–110 mmol/L |
| Potassium | 3.5–5.5 mmol/L | 3.6–5.9 mmol/L |
| Calcium | 2.2–2.6 mmol/L | 1.9–2.85 mmol/L |
| Magnesium | 0.75–1.0 mmol/L | 0.71–1.1 mmol/L |
| Phosphorus | 1.45–2.1 mmol/L | 1.4–3.0 mmol/L |
| Bicarbonate | 24–30 mmol/L | |
| Urea | 3–7 mmol/L | 1–5 mmol/L |
| Creatinine | 18–70 micromol/L | <20–150 micromol/L |
| Glucose | 3.9–6.9 mmol/L | |
| Osmolality | 275–295 mOsm/kg H 2O | 280–300 mOsmol/L |
| Anion gap | 5–12 mmol/L |
Electrolytes are obtained from the child’s diet in health (Martini Nath, 2009):
• Water is absorbed mostly by the process of osmosis, with sodium playing a major role.
• Glucose is co-transported with sodium with the help of insulin, and used by the cells to make energy.
• Amino acids also use a co-transport system together with sodium.
• Calcium, phosphate and sulphate absorption require active transport, accelerated by calcitriol and parathyroid hormone.
• Magnesium absorption requires carrier proteins.
• Chloride and bicarbonate are absorbed by diffusion or carrier-mediated transport.
• Potassium absorption in the small intestine is mainly by passive diffusion, and cellular uptake is influenced by insulin in activating the Na+K+ APTase pump.
Urea
Urea is a waste product of protein catabolism and is removed from the blood via the kidneys. Neonatal and childhood plasma urea concentration levels are lower than in adulthood, reflecting the heightened amino acid utilisation for protein synthesis in childhood. Adult values are reached between 10 and 12 years of age.
Creatinine
Creatinine in the blood is the product of muscle energy metabolism and thus has a direct relationship with muscle mass. Plasma creatinine levels, therefore, vary with the age and sex of the individual. Creatinine is cleared/excreted from the blood via the kidneys (Linné & Ringrud 1999, Walmsley & White 1994).
Glucose
Glucose is the simple sugar that is the immediate energy source for cell metabolism. It is the preferred source of energy for brain cells, which rely on an adequate blood glucose because the brain has very limited glycogen stores. Together with oxygen, glucose is used by cells to synthesise adenosine triphosphate (ATP), that provides the chemical energy for continuous effective cellular function.
The anion gap
The anion gap is the calculation of the mathematical difference between the anions chloride (Cl –) and bicarbonate (HCO 3–) & the cations sodium (Na +) and potassium (K +):
• [Cl – + HCO 3–] – [Na + + K +] = Anion Gap
• Normal value in children is 5–12 mmol/L (Davies & Hassell 2007)
• Raised levels [>14 or more] suggest metabolic acidosis as in diabetic ketoacidosis.
Blood urea and electrolytes assay, although invasive and therefore unpleasant for the child and family, is a valuable biological and clinical assessment tool whenever the integrity of blood biochemistry is in question, as in dehydration for example. The test estimates essential elements and the chemical components in the blood and urine. Depending on the child’s clinical state and technological facilities, the test result can be known within minutes, hours or days. Although reference values are offered here, individual units tend to operate on their laboratory-validated reference ranges that are calibrated to take into account the sources samples are obtained from and the machines used to carry out the assays. It is therefore the responsibility of all nurses to familiarise themselves with the accepted reference values in their own unit and to act promptly when results are outside these ranges.
Homeostasis (The regulation of body fluids and electrolytes) is a delicate, carefully orchestrated and balancing act between the hypothalamus and kidneys via a neurohormonal pathway regulates body fluids and electrolytes. The system works by a negative feedback in titrating intake and regulating output (see Tortora & Derrickson 2009).
The thirst mechanism
Thirst receptors present in the anterior hypothalamus are activated by low extracellular volume (hypothalamic.thirst) and high EC solute concentration (osmotic thirst). The receptors detect changes in both volume and osmolality of their immediate extracellular environment to create the sensation of thirst in the individual thus, water/fluid ingestion (Stricker & Verbalis 1999). Interestingly, even though a time lapse of 10–20 minutes exist for the ingested water/fluid to be absorbed and restore homeostasis, we never drink (water) ourselves to death. Here, in the phenomenon of oropharyngeal metering, the act of continuous swallowing (dipsogenic stimuli) forewarns the brain of the imminent restoration of CBV so the individual stops drinking before absorbing the ingested fluid/water (Figaro & Mack 1997). Figure 25.5 illustrates the behavioural response in body fluid and electrolyte homeostasis.
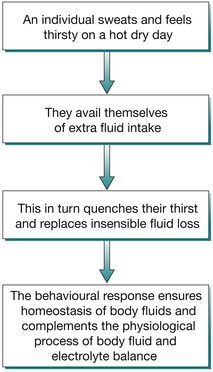 |
| Fig. 25.5 A behavioural response to increased insensible fluid loss on a hot day. |
Antidiuretic hormone
Adaptive changes in TBW and Na + balance are regulated by the anterior hypothalamus. Hypothalamic cells in close contact with the osmoreceptors are activated by decrease in CBV or increase in plasma osmolality to produce antidiuretic hormone (ADH), transported to and secreted by the posterior pituitary into the bloodstream. ADH on reaching the renal distal tubules and collecting ducts causes the synthesis and positioning of water channels (aquaporins) on the luminal side, making them very permeable to water. This enables them to reabsorb water from the tubules back into the bloodstream to restore CBV. (Martini Nath, 2009, Porterfield 2001).
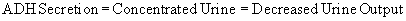 thus explaining the term “antidiuretic”.
thus explaining the term “antidiuretic”.
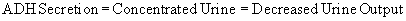
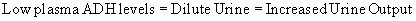
Clinically, appropriate ADH secretion exists if it is in response to high plasma osmolality or hypovolaemia. Here the osmostat is reset at a lower level (Cheethan & Baylis 2002). Alcohol acts as a diuretic by inhibiting ADH secretion.
Aldosterone
Aldosterone, secreted by the adrenal cortex works on the principal cells of the renal distal tubules and collecting ducts. Stimuli for their release include low CBV, autonomic stimulation of renin release, and a rise in plasma potassium level. It promotes renal tubular sodium reabsorption by increasing the synthesis and activity of sodium channels, and the Na +/K + ATPase pump. The increase in sodium reabsorption coincides with the excretion of potassium and hydrogen ions from the body in urine (Celsi & Aperia 1999). Caffeine acts as a diuretic by inhibiting the action of aldosterone on the renal tubules.
Conscientiousness and vigilance applies in managing fluid and electrolyte therapy in children, as these biological systems take time to acquire maturity (functional competence). Young children, especially infants, have impaired capacity to regulate body fluids and electrolytes due to the limited gain of control by the kidneys and a blunted response to the regulatory hormones (Guyton & Hall 2005, Holtbäck & Aperia 2003).
Dehydration in children
Children, especially infants, are at risk of body fluid and electrolyte imbalance. The reasons why children require a greater intake of water than adults are explained in Table 25.3. As the extracellular fluid is in direct contact with the outside world, and given that, the younger the child, the higher the proportion of extracellular fluid volume, any illness or situation in which fluid intake in the child is reduced or fluid is lost (e.g. acute diarrhoea and vomiting) creates potential for or actual imbalances (Elliot 2007, Endom et al 2008).
For example, a fever above 38˚C requires an increase in the child’s fluid intake of 10–12% because of the increased radiant heath, and thus water, loss. Tachypnoea similarly increases the child’s fluid requirement two- to three-fold. Children undergoing surgery (because of the period of fasting involved and anaesthesia-related vasodilation), those on cancer chemotherapy, those who sustain moderate to severe burns and scalds, or present with vasco-occlusive sickle-cell painful crisis are all at risk of body fluid and electrolyte imbalance. Finally, any major systemic disease or trauma, and limited paediatric knowledge and expertise in both nursing and medical practice, can increase the risk of body fluid and electrolyte imbalance in children. Thus, it is important that when a child enters into a healthcare setting, the people caring for that child know and care about what they are doing, highlighting possible inadvertent care deficits and attendant risks in managing the child’s fluid and electrolyte requirement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


