Chapter 30. Caring for children
the role of the immune system in protecting against disease
Maureen R. Harrison
LEARNING OUTCOMES
• Be able to understand the different phases of the immune response.
• To identify how innate immunity provides the first line of defence in protecting against infections.
• Be aware of the physiological barriers that prevent infection occurring.
• To understand other factors of first-line defence, such as cell-mediated and humoral immunity, which enable most infants and children to respond to microbial attack.
• To apply knowledge of innate immunology to principles of nursing practice, in particular infection control.
• To outline the assessment of a child with acute infection.
Introduction
The efficiency of the immune system in protecting against infection is often taken for granted in countries such as the UK. Even the World Health Organization (WHO) has demonstrated that, in developed countries like the UK, death from respiratory infections is not as significant as other causes of death, being only fourth in ranking from the ten leading causes of death in high income countries (WHO 2008). However, in low income countries, six of the top ten causes of death have infectious and perinatal causes. Figure 30.1 demonstrates causes of death among children under the age of 5 worldwide. It is important to note that 54% of deaths are also associated with malnutrition.
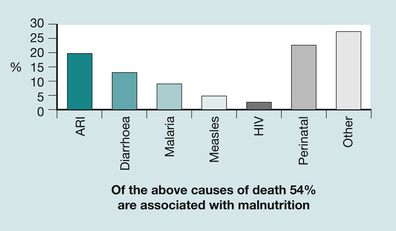 |
| Fig. 30.1 Proportional mortality among under fives worldwide, 2001. Of the causes of death shown, 54% of deaths are associated with malnutrition (reproduced with permission from WHO 2003). |
A limited number of viral and bacterial pathogens are responsible for infectious disease among neonates and infants. The most pathogenic bacteria are encapsulated bacteria, such as Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, Escherichia coli, Neisseria meningitidis, and Haemophilus influenza (Klouwenberg & Bont 2008) and immunity against these organisms protects an infant from disease. An understanding of the nature of the battle between humans and microorganisms, and the role of the immune system in protecting children from infectious diseases, is important. This knowledge then needs to be linked to how the children’s nurse can promote and optimise health.
Many infectious agents (bacteria, viruses, fungi and protozoa) would use any opportunity to utilise the human body as a means for growth and proliferation. However, the human body has developed a series of very effective defence mechanisms to establish immunity against infection (Roitt et al 2006). The word ‘immunity’ comes from the Latin word immunitas, meaning ‘freedom from’. In most instances, the human body is very efficient at avoiding attack but at certain times the infectious agents win, the result being the development of an infection (Roitt et al 2006).
There are many interindividual variations in how the immune system functions such as genetics, age, gender, diet, history of infections and vaccination (Clader & Kew 2002), and Figure 30.1 demonstrates the importance of nutrition; however, this chapter is concerned predominantly with the main process of immunity. The immune system has three phases in the overall response to attack:
• Phase 1: immediate response – innate immunity
• Phase 2: the inflammatory response
• Phase 3: the adaptive immune response.
Phase 1. The immediate response to infection: innate immunity
At birth, most infants have the capacity to develop a very efficient immune system, as is demonstrated by the majority of infants who survive their first year of life without suffering from major life-threatening infections. Indeed, from as early as 20–26 gestational weeks, the fetus has been observed to be capable of mounting a sophisticated immune response, as evidenced by those fetuses who were infected with rubella during pregnancy (Warner 2004) and were shown to have antibodies against the rubella in their blood. However, the immune system is not fully developed at birth, and certain ‘units’ need to undergo learning programmes that will eventually enable them to recognise and destroy the huge dimension of immunogens (agents that are harmful to the human host) (Stiehm 1996). That part of the immune system involved in learning is referred to as the ‘adaptive immune response’ and includes the development of specific immune cells, lymphocytes and the production of antibodies (immunoglobulins) against all harmful microorganisms. This arm of the immune system will be not be discussed in any depth in this chapter, although aspects of it will be referred to.
The first means of defence against microorganisms that would cause harm to the body is called ‘innate immunity’ or ‘non-specific immunity’ and previous exposure to these organisms is not needed to provide protection against infection. In other words, this part of the immune system does not need to learn how to destroy or render inactive these organisms because the ability to know how to fight against them is inherent, and from birth most babies have these fundamental immunological capabilities. The three main mechanisms of innate immunity are (Fig. 30.2):
• barrier mechanisms
• cell-mediated mechanisms
• humoral mechanisms.
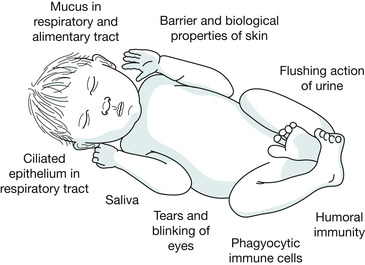 |
| Fig. 30.2 The key features of innate immunity. |
These non-specific resistance mechanisms are responsible for the major part (> 95%) of host defence, the ‘host’ being the human body; they will therefore be studied in detail in this chapter (Zinkernagel 2003).
Barrier mechanisms
Many of the essential components of the immune system develop very early in fetal life and most healthy newborn babies are prepared to deal with antigen challenges (Janeway et al., 2004 and Newell and McIntyre, 2000). An antigen is any molecule that can stimulate an immune response, in particular a response that mobilises the adaptive immune system. The first lines of defence against infection are the external systems, which are commonly described as the barrier mechanisms. These are so called because they protect individuals by stopping harmful microorganisms from entering the body, and inhibit colonisation of the same.
Skin
The skin is the largest organ in the young baby and it provides an intact, dry surface that is resistant to the penetration of microorganisms. Not all microorganisms are harmful and the skin of children and adults is normally host to a number of bacteria, such as harmless diphtheriods, Gram-positive cocci and yeasts, which are called commensal microorganisms (Greenwood et al 2002). They are called ‘commensal’ because they have been ‘permitted’ to live on the skin and they provide some protection by taking up space and competing for nutrients that cannot then be used by more harmful disease-causing bacteria. They also produce by-products that inhibit the growth of other organisms (Greenwood et al 2002).
At birth, the skin is sterile because it has been contained within amniotic fluid. A biological advantage of an infant being nursed in close skin-to-skin contact with the mother immediately after birth is to encourage the rapid colonisation of the infants previously sterile skin, with the mothers harmless commensal bacteria and fungi (Inglis 2003).
Nurses must remember that they also carry commensal bacteria on their skin. In most instances the bacteria on their skin will remain harmless if transferred to children by contact. However, stringent infection control measures are paramount to reduce transfer of potentially harmful pathogens through skin contact (RCN 2009).
 Activity
Activity
 Activity
ActivityReview the following online course on infection prevention, in particular with relation to hand washing:
Also go through the publication ‘The management and control of hospital acquired infections in acute NHS Trusts in England’ (Department of Health (DoH), 2003 and Department of Health, 2006):
Analyse the implications for your own practice.
The Health Protection Agency (2008) has identified that the very young are particularly vulnerable to hospital acquired infections (HAI) and that most infections were associated with hospital strains of MRSA (methicillin-resistant Staphyloccoccus aureus). Hand-washing has proven to be one of the most effective preventative measures against HAIs (Bissett, 2003, Parker, 1999 and Ward, 2000).
 Scenario
Scenario
 Scenario
ScenarioKhaleda has been admitted with infected atopic dermatitis and microbiology reports indicate that her skin is infected with MRSA:
• From your understanding of the barrier properties of innate immunity, identify why she is more prone to developing an infection in her skin.
• Discuss how you will manage her care. To inform your discussion these are topics you must address:
• infection control procedures
• risk factors for developing MRSA
• the rationale behind presenting symptoms
• treatment strategies
• the evidence base for treatment.
Non-compliance with hand-washing policies is a well-known problem and Ng et al (2004) found that a technique of hand rub with alcohol preparations and the wearing of non-sterile gloves led to a considerable reduction in infection episodes. Although this procedure can be monitored more overtly than straightforward hand-washing, it cannot fully replace the need to adequately wash the hands before any procedure involving an infant and child.
Serious bacterial infections in infants are not solely attributable to hand-washing and the mode for infection spread can also include nasopharyngeal colonisation and through penetration of microorganisms into the blood (Brook 2003). These organisms can gain access to the blood through microscopic tears in the skin, such as those caused by intravenous access devices (Inglis 2003). Other organisms cited as responsible for early onset neonatal sepsis are the group B streptococcus, Escherichia coli and Staphylococcus epidermidis, all of which are both commensal and nosocomial organisms, and most of which can be eliminated by meticulous hand-washing (Ballot 2000, Nenstiel et al 1997).
The skin controls the numbers of commensal bacteria through various secretions such as sweat and sebum. There are two types of sweat gland, one of which – the eccrine glands – are functional at birth. Apocrine sweat glands, which are found mainly in the axillae, groin, face and scalp are stimulated by the sex hormones and therefore do not become fully functional until puberty (McCance & Huether 2005). Sweat contains mostly water, salts, traces of metabolic wastes and some immunoglobulin A (IgA). Immunoglobulins are produced by B lymphocytes, very specialised immune cells and part of the adaptive immune system, which are produced from the lymphoid lineage in the bone marrow (Parham 2005). In the newborn infant, IgA antibodies in sweat are quite limited, but fortunately the concentration of salt and a fairly acidic pH 4–6 is inhibitory or lethal to the survival of many microorganisms (Rennie & Roberton 1999, Whaley & Wong 1995).
The sebaceous glands, which also produce sebum, are stimulated by maternal androgens and become very active in the last trimester and early infancy. In utero, these produce vernix caseosa, a white, waxy substance that gives an additional layer of protection in terms of its antimicrobial and mechanical properties (Greenwood et al., 2002 and Marchini et al., 2002). Following the birth of the baby, the mother is advised not to wipe away vernix caseosa because not only does it protect the skin against microorganism attack, it is one of nature’s most efficient emollients. Many mothers worry about the white spots on their babies faces and they need to be assured that these are not acne but the final remnants of vernix in the skin pores.
The skin is also protected by mechanical means, which is the thickness of the skin and the hardness of keratinised cells, however the delicate neonate’s skin lacks the hard extra layers of the skin of an older child. In the very low birthweight infant the stratum corneum is absent or deficient (Bautista et al., 2000 and Marchini et al., 2002), therefore awareness of these infants’ lack of the immunological barrier properties of the skin is crucial.
In fully developed skin, cells divide rapidly, the top layers constantly being renewed and dead cells sloughing away. An example of how proliferate the sloughing action is can be seen in the dust that relentlessly collects on furniture, most of which is dead skin cells. This sloughing action provides protection, because any harmful bacteria that are starting to colonise the skin will be sloughed away (Janeway et al 2004). Regular cleansing of an infant and child’s skin not only facilitates the sloughing action but the massaging action stimulates the blood supply to the skin, which protects the skin immunologically and encourages growth of new cells.
 Activity
Activity
 Activity
Activity
 Activity
ActivityBasic hygiene care
Prepare written guidelines for new parents on the hygiene needs and principles of care for their baby. Identify actions required in a step-by-step approach and provide a simple rationale for each action taken.
 Activity
ActivityReflecting on practice
Review the guidelines in caring for the skin of premature and low birthweight infants in Storm & Lund Jensen (1999):
1. Due to the immaturity of the skin of the preterm infant, it is particularly susceptible to damage.
2. Adhesives for attaching probes, etc., should be used as little as possible and should be removed with great care.
3. Bathing is not recommended for very low birthweight babies for at least 7 days after birth.
Are there variations in the way these recommendations have been implemented in your experience of neonatal care?
Cleansing is of particular importance in the nappy area, because the combination of microorganisms and urine together in a damp environment will encourage the growth of certain organisms, and in turn, their by-products will contribute to skin breakdown.
The final means of the skin providing protection is through Langerhans cells (immature dendritic cells) and macrophages, which are found in the dermis. These cells arise from the myeloid lineage of cells produced in the bone marrow (Parham 2005), all of the cells from this line providing innate protection. The role of dendritic cells, Langerhans cells and macrophages is to recognise, ingest and destroy antigens, presenting them later to T and B lymphocytes, for future recognition. Most of the cells from the myeloid lineage release lysozymes and cytokines from granules within their cytoplasm. Lysozymes are enzymes that damage and cause the death of bacteria by splitting the sugars found in their cell walls, thus destroying the bacterial cell wall. Cytokines will be discussed later but they are protein messengers that affect the behaviour of other cells (Parham 2005).
Like all soldiers, Langerhans cells need to be fully equipped for killing and to have the ability to recognise the enemy before they can attack. Mature Langerhans cells, which are ready for attack, can be found from the 5th month of gestation, but some of their action is limited until well after birth, owing to their lack of opportunity to have previously met and therefore recognised antigens in utero (Ballot 2000, Rennie & Roberton 1999). This final means of control is vital, especially when the top epidermal layer of the skin is broken by small cuts or erosions, and it is another reason why nurses must be aware that skin damage in a very young baby, even through a tiny venepuncture site, will render the infant more prone to attack, invasion from microorganisms and the development of infection (Janeway et al 2004).
A summary of the protective properties of skin is shown in Box 30.1.
 Box 30.1
Box 30.1 Summary of protective properties of skin
• Intact skin resistant to microorganisms
• Presence of commensal organisms
• Skin secretions such as sweat and sebum
• Thickness of skin
• Sloughing off of old skin cells and rapid replacement of new skin cells
• Presence of immune cells within the dermis of skin
• Presence of immune proteins such as antibodies and complement
Respiratory tract
Mucous lines the surface of many areas in the body, such as the respiratory tract. To some extent, these areas are more prone to infection than skin, because their moist and sticky surface provides a good breeding environment to bacteria (Janeway et al 2004). In the respiratory tract, microorganisms and unwanted dust particles that have been inhaled are trapped in the mucus, which is constantly being driven upwards, by the action of cilia, towards the pharynx. The mucus, with all its debris, is then either coughed out or swallowed. Cough and swallow reflexes have been demonstrated as early as 12 weeks’ gestation (McIntosh et al 2003), and are therefore well developed in a full-term healthy neonate. These reflexes are both efficient means of expelling unwanted invaders to the body.
However, the ability of the entire respiratory tract to produce mucus is reduced in infancy (McIntosh et al 2003) and breach of this fine layer of mucus through a number of factors (Rona, 2000, Schwartz, 2004 and von Mutius, 2000) will cause a breach in the protective properties and render the infant very prone to colonisation by airborne microorganisms or antigens (Janeway et al 2004). Viruses in particular are very quick to take advantage of this reduced protection in the young baby, hence the relatively frequent occurrence of symptoms of ‘snuffles’, caused in response to viral attack.
The antibody immunoglobulin A (IgA) is secreted into the mucous linings. Two of the roles of IgA are to inhibit the adherence and proliferation of any antigens, such as bacteria, on epithelial surfaces. In the infant, IgA is one of the last immunoglobins to reach competent levels, therefore the neonate has relatively less protection against infection from those parts of the body where microorganisms can gain such easy entry, such as the respiratory tract (Newell & McIntyre 2000). However, IgA protection can be provided from the mother as discussed later.
It has been found that those children who have enhanced responsiveness to allergens (produce more immunoglobulins) in the neonatal period (Warner 2004) have been associated with a later risk of developing allergies such as asthma. These infants produce IgE instead of IgA. Subsequent exposure to the allergen that has resulted in the production of IgE, causes binding of the allergen to the IgE on the mast cell. This triggers the inflammatory response, which can vary in severity and, in some circumstances, produce life-threatening reactions such as severe asthma or anaphylaxis. Unfortunately, the damage caused by the allergic reaction is out of proportion to the threat posed by the allergen.
Eyes
The eyes are potentially an area where microorganisms can gain access into the body. Protection is provided by the conjunctiva, which is covered by a film of tears, and the constant blinking of the eyes flushes and cleanses the eye surface. Tears contain antibacterial products such as lysozyme but tear glands do not normally function until the child is 2–4 weeks old. Because of their narrow lumen, the tear glands are liable to blockage, which prevents the properties of tears from washing the eyes and renders the small baby prone to invasion by microorganisms that can cause conjunctivitis (Greenwood et al., 2002 and Kreir and Mortensen, 1990). Parents should be shown how to clean their babies eyes by taking a damp piece of soft material from the area nearest the nose, the caruncle, and then across to the other side. This action helps to clear the tear ducts.
The gastrointestinal tract
The large surface area of the gastrointestinal tract is covered by a single layer of columnar epithelium and mucus, which allows for absorption of nutrients but also for entry of microbial pathogens (Garside & Mowat 1999). Within moments of birth, the gastrointestinal tract is challenged by myriads of bacterial and food antigens. There is evidence to suggest that even before birth the gastrointestinal tract is exposed to some antigen challenges as the fetus swallows amniotic fluid (Warner 2004). These swallowed antigens are met by antigen-presenting cells (APCs, e.g. macrophages and dendritic cells) in the fetus’ gastrointestinal tract. For lymphocytes from the adaptive immune system to recognise antigens, they must first be presented by these antigen-presenting cells and the gastrointestinal tract is one of the only organs in the newborn infant with mature antigen-presenting cells.
The adaptive immune system, which allows for the formation of immune memory, is prominent at the mucosal site within the gastrointestinal tract. There are two subsets of memory T cells, natural and acquired. The natural memory T cells are self-specific, responding to self-antigens such as stressed and worn out epithelial cells. The acquired memory T cells gain their memory from antigens presented by antigen-presenting cells (Cheroutre & Madakamutil 2004). They then stimulate B cells to produce immunoglobulins specific to the antigens, such as IgA and IgG. In most cases, the presence of those immunoglobulins induces tolerance to those antigens within the gastrointestinal tract (Warner 2004) by neutralising the antigen and rendering it harmless. Unfortunately, there are instances when the antigen introduced to the gastrointestinal tract becomes immunogenic, meaning that it will cause a reaction including inflammation of the local tissues within the gastrointestinal tract. The result of this reaction is once again because of the production of IgE rather than IgA and IgG. Examples of this are those babies who develop intolerance to the proteins, carbohydrates or fats in manufactured baby milks, or the immune responses to gluten in patients with coeliac disease.
 Seminar discussion topic
Seminar discussion topic
 Seminar discussion topic
Seminar discussion topicRead the article by Sicherer (2003). This article discusses a range of food allergies seen in infants and children:
• Discuss the common symptoms demonstrated by food allergies.
• Differentiate between what is an allergy and a hypersensitivity.
Many food intolerances do resolve in time but management of children’s diets can be a problem for parents. Choose a couple of the more common disorders and identify what the problems might be and the advice you would give to parents regarding dietary management.
There is a huge network of immune tissue called gut-associated lymphoid tissue (GALT), which acts as a selective barrier to prevent the entry of these antigenic or infectious material into the body. The gastrointestinal tract is therefore considered the largest ‘immune organ’ in the body, with more than 70% of immune cells, both innate and adaptive, located there (Garside and Mowat, 1999 and Grönlund et al., 2000).
Neonates have a limited amount of GALT but this develops rapidly over the first 2 years of life and certainly feeding is associated with the maturation of gut-associated lymphoid tissue (Flidel-Rimon et al 2004). Immunological protection is also provided by secretory IgA, which is quantitatively and functionally defective for a variable period after birth (Flidel-Rimon et al 2004). The number of IgA plasma cells (B lymphocytes) in the duodenal mucosa reaches that of adults by 2 years of age, whereas the level of mucosal secretory IgA antibodies reaches adult levels only at the age of 6–8 years (Grönlund et al 2000). Owing to the undeveloped mucosal immunological protection, there is higher vulnerability to infection and possibly more sensitisation to dietary antigens (MacDonald, 1994 and Warner, 2004).
The saliva in the mouth has protective properties, which include IgA, lysozymes, mucopolysaccharides, which block some viruses, and glycolipids that compete with bacterial cell wall products for attachment to the mucous membranes (Greenwood et al., 2002 and Janeway et al., 2004).
The newborn infant is unable to gain the full protective benefits of saliva because saliva production is limited in comparison to the amount produced later in life. However, this is compensated for as the different shape of the mouth and oral cavity facilitates efficient sucking and allows milk to be swallowed almost immediately. As milk does not stay in the mouth long, saliva is not needed for its protective action. Breastfeeding counteracts most of the immunological deficiencies because breast milk passively prevents infections getting into and crossing the intestinal barrier, and it also promotes the infant’s immunity (Filteau 2000). For example the IgA concentrations found in breast milk are very high in the first few months of feeding (Weaver et al 1998). Other protective properties of breast milk include lysozyme and lactoferrin, which is an iron-binding protein that has a bactericidal effect on E. coli. Breast milk also contains macrophages and lymphocytes, which play a vital role in the immune response (Cushing et al., 1998, Howie et al., 1990, Oddy et al., 2003 and Wilson et al., 1998, WHO 2002).
Early introduction of enteral feeding in preterm infants is recommended (Flidel-Rimon et al., 2004 and Okada et al., 1998) owing to the reduced risk of nosocomial infection such as neonatal sepsis, maintenance of the intestinal barrier and development of competent mucosal immunity.
The production of saliva is only noticeably increased around 4 months. Around about this time, as the child starts weaning, the shape of the mouth changes, food is kept in it for longer periods and production of saliva is noticeably increased through the baby drooling (Weaver et al 1998




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
